Abstract
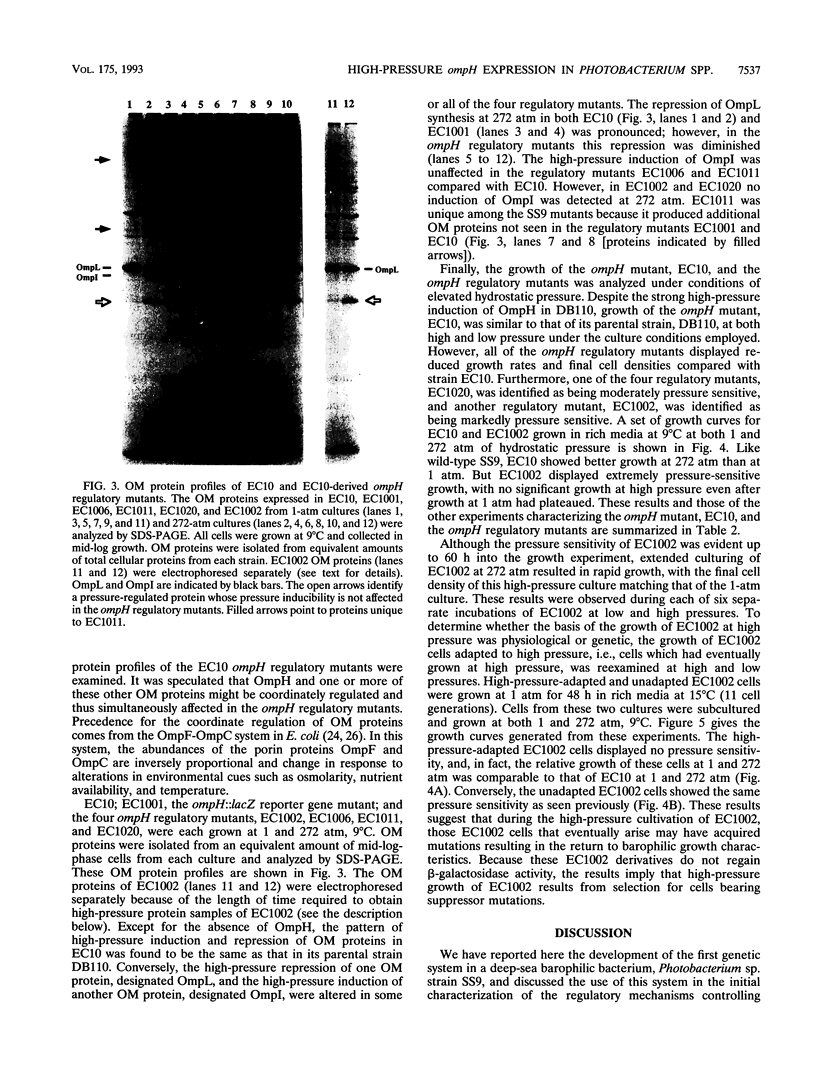
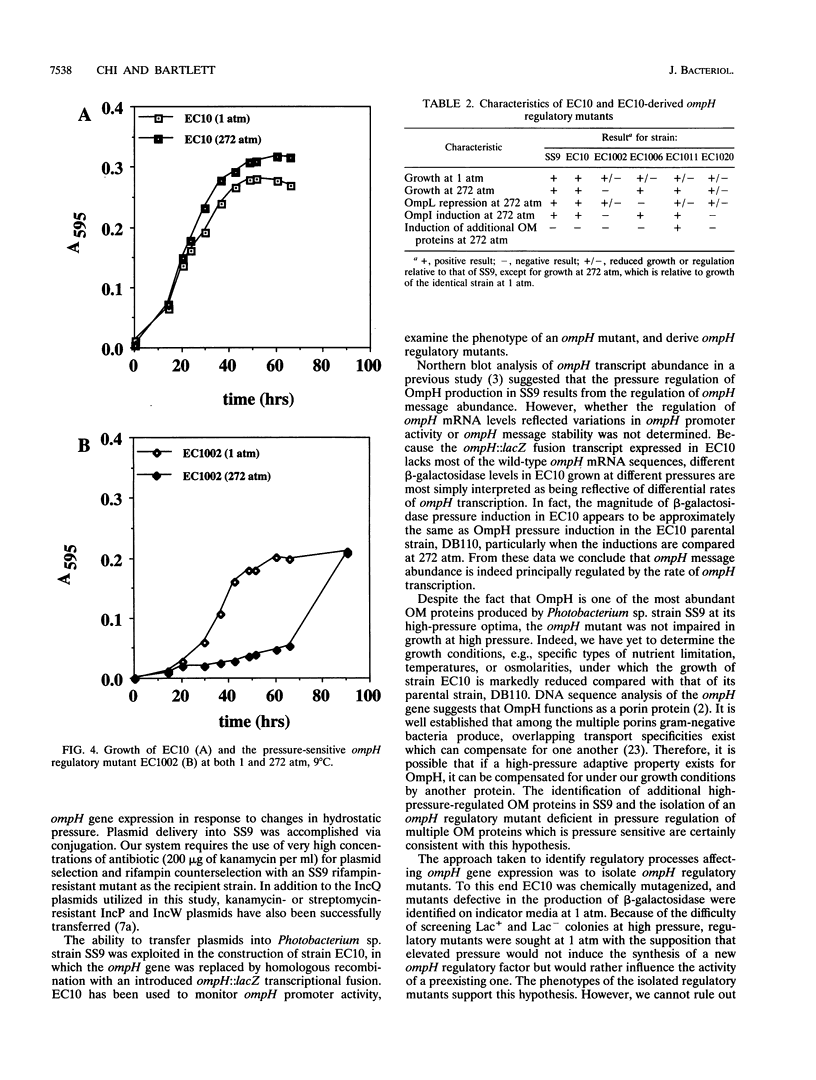
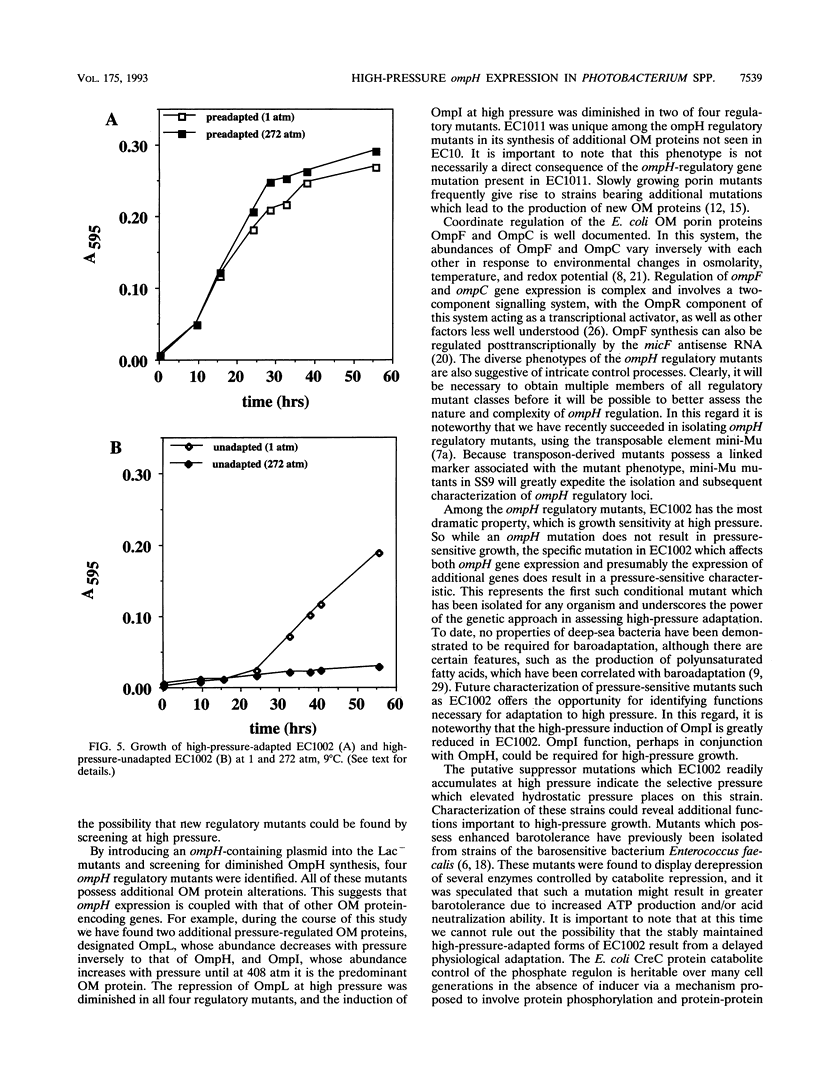
Photobacterium sp. strain SS9 is a deep-sea bacterium which modulates the abundances of several outer membrane proteins as a function of hydrostatic pressure. These proteins include the product of the previously cloned ompH gene (D. H. Bartlett, M. Wright, A. A. Yayanos, and M. Silverman. Nature (London) 342:572-574, 1989). Subsequent to conjugal plasmid delivery it was possible to cross an ompH::lacZ transcriptional fusion into the genome of SS9, replacing the wild-type ompH gene, generating strain EC10. EC10 is not impaired in growth at high pressure, indicating that under the growth conditions employed, OmpH is not required for baroadaptation. beta-Galactosidase production in EC10 is induced by high pressure to approximately the same extent that OmpH production is in the parental strain, SS9. Therefore, OmpH abundance appears to be primarily regulated at the transcriptional level. EC10 was used for the isolation of ompH regulatory mutants. Derivatives of EC10 which produce reduced levels of beta-galactosidase at both low and high pressure and which appeared to possess mutations outside the ompH::lacZ locus were obtained. All of these regulatory mutants displayed alterations in the high-pressure repression of a second outer membrane protein, designated OmpL, and two of the mutants were also deficient in the high-pressure induction of a third outer membrane protein, designated OmpI. The most dramatic phenotype was present in mutant EC1002, whose growth was extremely barosensitive. EC1002 is the first pressure-sensitive mutant ever isolated. Prolonged incubation of EC1002 at high pressure led to the accumulation of cells with wild-type growth characteristics at high pressure. These cells are suggested to possess suppressor mutations, as they remain deficient in beta-galactosidase production and maintain their high-pressure-adapted phenotype for many generations in the absence of high-pressure selection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Bartlett D. H., Chi E., Wright M. E. Sequence of the ompH gene from the deep-sea bacterium Photobacterium SS9. Gene. 1993 Sep 6;131(1):125–128. doi: 10.1016/0378-1119(93)90680-2. [DOI] [PubMed] [Google Scholar]
- Bartlett D., Wright M., Yayanos A. A., Silverman M. Isolation of a gene regulated by hydrostatic pressure in a deep-sea bacterium. Nature. 1989 Nov 30;342(6249):572–574. doi: 10.1038/342572a0. [DOI] [PubMed] [Google Scholar]
- Better M., Helinski D. R. Isolation and characterization of the recA gene of Rhizobium meliloti. J Bacteriol. 1983 Jul;155(1):311–316. doi: 10.1128/jb.155.1.311-316.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Campbell J., 3rd, Bender G. R., Marquis R. E. Barotolerant variant of Streptococcus faecalis with reduced sensitivity to glucose catabolite repression. Can J Microbiol. 1985 Jul;31(7):644–650. doi: 10.1139/m85-121. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. H., Greenberg J. T., Demple B. Posttranscriptional repression of Escherichia coli OmpF protein in response to redox stress: positive control of the micF antisense RNA by the soxRS locus. J Bacteriol. 1993 Feb;175(4):1026–1031. doi: 10.1128/jb.175.4.1026-1031.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong E. F., Yayanos A. A. Adaptation of the membrane lipids of a deep-sea bacterium to changes in hydrostatic pressure. Science. 1985 May 31;228(4703):1101–1103. doi: 10.1126/science.3992247. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J., Chai T. J. New major outer membrane proteins found in an Escherichia coli tolF mutant resistant to bacteriophage TuIb. J Bacteriol. 1978 Mar;133(3):1478–1483. doi: 10.1128/jb.133.3.1478-1483.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Siehnel R., Martin N. Outer membrane proteins of Pseudomonas. Mol Microbiol. 1990 Jul;4(7):1069–1075. doi: 10.1111/j.1365-2958.1990.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Henning U., Schmidmayr W., Hindennach I. Major proteins of the outer cell envelope membrane of Escherichia coli K-12: multiple species of protein I. Mol Gen Genet. 1977 Sep 9;154(3):293–298. doi: 10.1007/BF00571285. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marquis R. E., Bender G. R. Isolation of a variant of Streptococcus faecalis with enhanced barotolerance. Can J Microbiol. 1980 Mar;26(3):371–376. doi: 10.1139/m80-060. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R., Jr, Tolan R. W., Jr Molecular cloning, expression, and primary sequence of outer membrane protein P2 of Haemophilus influenzae type b. Infect Immun. 1989 Jan;57(1):88–94. doi: 10.1128/iai.57.1.88-94.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Porins and specific channels of bacterial outer membranes. Mol Microbiol. 1992 Feb;6(4):435–442. doi: 10.1111/j.1365-2958.1992.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]