Abstract
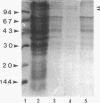
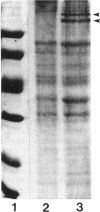
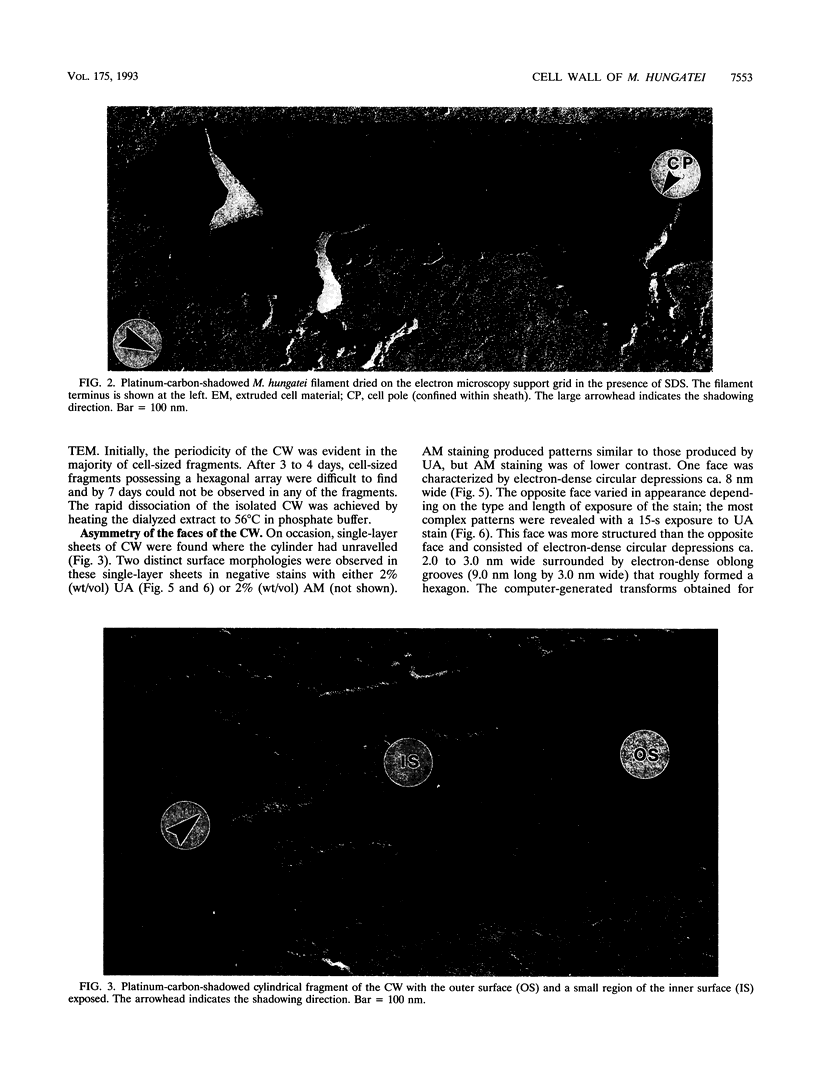
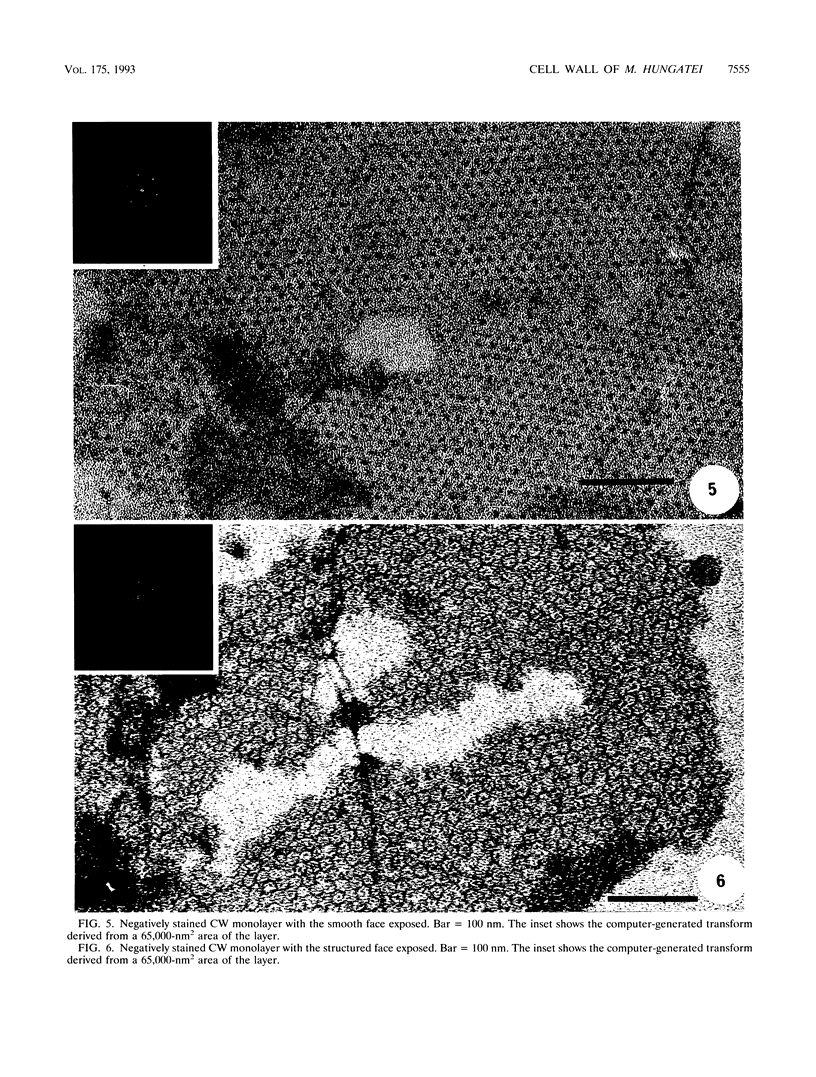
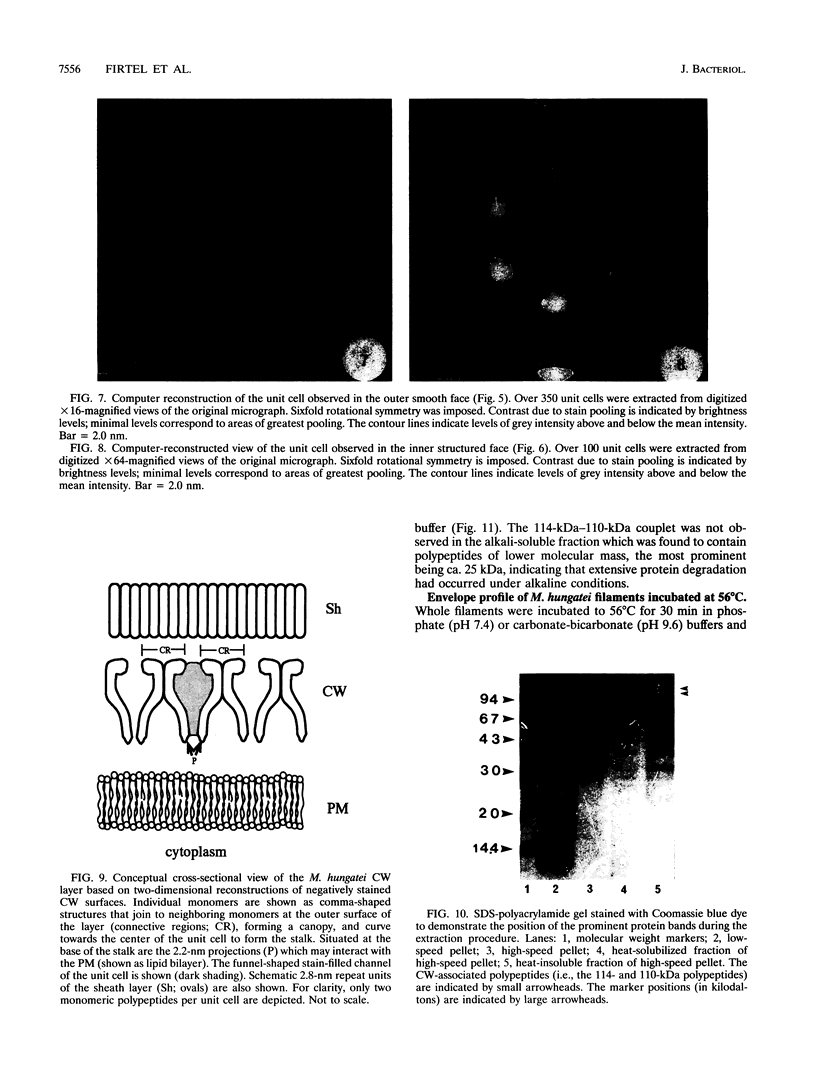
The cell wall of Methanospirillum hungatei GP1 is a labile structure that has been difficult to isolate and characterize because the cells which it encases are contained within a sheath. Cell-sized fragments, 560 nm wide by several micrometers long, of cell wall were extracted by a novel method involving the gradual drying of the filaments in 2% (wt/vol) sodium dodecyl sulfate and 10% (wt/vol) sucrose in 50 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES) buffer containing 10 mM EDTA. The surface was a hexagonal array (a = b = 15.1 nm) possessing a helical superstructure with a ca. 2.5 degrees pitch angle. In shadowed relief, the smooth outer face was punctuated with deep pits, whereas the inner face was relatively featureless. Computer-based two-dimensional reconstructed views of the negatively stained layer demonstrated 4.0- and 2.0-nm-wide electron-dense regions on opposite sides of the layer likely corresponding to the openings of funnel-shaped channels. The face featuring the larger openings best corresponds to the outer face of the layer. The smaller opening was encircled by a stalk-like mass from which 2.2-nm-wide protrusions were resolved. The cell wall in situ was degraded at pH 9.6 at 56 degrees C but was unaffected at pH 7.4 at the same temperature. The cell wall was composed of two nonglycosylated polypeptides (114 and 110 kDa). The cell wall resembled an archaeal S layer and may function in regulating the passage of small (< 10-kDa) sheath precursor proteins.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumeister W., Wildhaber I., Phipps B. M. Principles of organization in eubacterial and archaebacterial surface proteins. Can J Microbiol. 1989 Jan;35(1):215–227. doi: 10.1139/m89-034. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Choquet C. G., Patel G. B., Sprott G. D. Freeze-fracture planes of methanogen membranes correlate with the content of tetraether lipids. J Bacteriol. 1993 Feb;175(4):1191–1197. doi: 10.1128/jb.175.4.1191-1197.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J., Sprott G. D., Whippey P. Ultrastructure, inferred porosity, and gram-staining character of Methanospirillum hungatei filament termini describe a unique cell permeability for this archaeobacterium. J Bacteriol. 1991 Jan;173(1):130–140. doi: 10.1128/jb.173.1.130-140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J., Stewart M., Doyle R. J., Sprott G. D. Unusual stability of the Methanospirillum hungatei sheath. J Bacteriol. 1985 May;162(2):728–737. doi: 10.1128/jb.162.2.728-737.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröckl G., Behr M., Fabry S., Hensel R., Kaudewitz H., Biendl E., König H. Analysis and nucleotide sequence of the genes encoding the surface-layer glycoproteins of the hyperthermophilic methanogens Methanothermus fervidus and Methanothermus sociabilis. Eur J Biochem. 1991 Jul 1;199(1):147–152. doi: 10.1111/j.1432-1033.1991.tb16102.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Graham L. L., Beveridge T. J. Evaluation of freeze-substitution and conventional embedding protocols for routine electron microscopic processing of eubacteria. J Bacteriol. 1990 Apr;172(4):2141–2149. doi: 10.1128/jb.172.4.2141-2149.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovmöller S., Sjögren A., Wang D. N. The structure of crystalline bacterial surface layers. Prog Biophys Mol Biol. 1988;51(2):131–163. doi: 10.1016/0079-6107(88)90012-0. [DOI] [PubMed] [Google Scholar]
- Kist M. L., Murray R. G. Components of the regular surface array of Aquaspirillum serpens MW5 and their assembly in vitro. J Bacteriol. 1984 Feb;157(2):599–606. doi: 10.1128/jb.157.2.599-606.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval S. F., Jarrell K. F. Ultrastructure and biochemistry of the cell wall of Methanococcus voltae. J Bacteriol. 1987 Mar;169(3):1298–1306. doi: 10.1128/jb.169.3.1298-1306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lechner J., Sumper M. The primary structure of a procaryotic glycoprotein. Cloning and sequencing of the cell surface glycoprotein gene of halobacteria. J Biol Chem. 1987 Jul 15;262(20):9724–9729. [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L. Purification and characterization of a prokaryotic glucoprotein from the cell envelope of Halobacterium salinarium. J Biol Chem. 1976 Apr 10;251(7):2005–2014. [PubMed] [Google Scholar]
- Messner P., Pum D., Sára M., Stetter K. O., Sleytr U. B. Ultrastructure of the cell envelope of the archaebacteria Thermoproteus tenax and Thermoproteus neutrophilus. J Bacteriol. 1986 Jun;166(3):1046–1054. doi: 10.1128/jb.166.3.1046-1054.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner P., Sleytr U. B. Crystalline bacterial cell-surface layers. Adv Microb Physiol. 1992;33:213–275. doi: 10.1016/s0065-2911(08)60218-0. [DOI] [PubMed] [Google Scholar]
- Pangborn J., Starr M. P. Ultrastructure of Lampropedia hyalina. J Bacteriol. 1966 May;91(5):2025–2030. doi: 10.1128/jb.91.5.2025-2030.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel G. B., Roth L. A. Effect of sodium chloride on growth and methane production of methanogens. Can J Microbiol. 1977 Jul;23(7):893–897. doi: 10.1139/m77-131. [DOI] [PubMed] [Google Scholar]
- Patel G. B., Roth L. A., van den Berg L., Clark D. S. Characterization of a strain of Methanospirillum hungatti. Can J Microbiol. 1976 Sep;22(9):1404–1410. doi: 10.1139/m76-208. [DOI] [PubMed] [Google Scholar]
- Phipps B. M., Huber R., Baumeister W. The cell envelope of the hyperthermophilic archaebacterium Pyrobaculum organotrphum consists of two regularly arrayed protein layers: three-dimensional structure of the outer layer. Mol Microbiol. 1991 Feb;5(2):253–265. doi: 10.1111/j.1365-2958.1991.tb02106.x. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Schultze-Lam S., Harauz G., Beveridge T. J. Participation of a cyanobacterial S layer in fine-grain mineral formation. J Bacteriol. 1992 Dec;174(24):7971–7981. doi: 10.1128/jb.174.24.7971-7981.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southam G., Beveridge T. J. Detection of growth sites in and protomer pools for the sheath of Methanospirillum hungatei GP1 by use of constituent organosulfur and immunogold labeling. J Bacteriol. 1992 Oct;174(20):6460–6470. doi: 10.1128/jb.174.20.6460-6470.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southam G., Beveridge T. J. Dissolution and immunochemical analysis of the sheath of the archaeobacterium Methanospirillum hungatei GP1. J Bacteriol. 1991 Oct;173(19):6213–6222. doi: 10.1128/jb.173.19.6213-6222.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southam G., Firtel M., Blackford B. L., Jericho M. H., Xu W., Mulhern P. J., Beveridge T. J. Transmission electron microscopy, scanning tunneling microscopy, and atomic force microscopy of the cell envelope layers of the archaeobacterium Methanospirillum hungatei GP1. J Bacteriol. 1993 Apr;175(7):1946–1955. doi: 10.1128/jb.175.7.1946-1955.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprott G. D., Colvin J. R., McKellar R. C. Spheroplasts of Methanospirillum hungatii formed upon treatment with dithiothreitol. Can J Microbiol. 1979 Jun;25(6):730–738. doi: 10.1139/m79-106. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., McKellar R. C. Composition and properties of the cell wall of Methanospirillum hungatii. Can J Microbiol. 1980 Feb;26(2):115–120. doi: 10.1139/m80-017. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., Shaw K. M., Jarrell K. F. Isolation and chemical composition of the cytoplasmic membrane of the archaebacterium Methanospirillum hungatei. J Biol Chem. 1983 Mar 25;258(6):4026–4031. [PubMed] [Google Scholar]
- Stewart M., Beveridge T. J., Sprott G. D. Crystalline order to high resolution in the sheath of Methanospirillum hungatei: a cross-beta structure. J Mol Biol. 1985 Jun 5;183(3):509–515. doi: 10.1016/0022-2836(85)90019-1. [DOI] [PubMed] [Google Scholar]
- Sára M., Sleytr U. B. Molecular sieving through S layers of Bacillus stearothermophilus strains. J Bacteriol. 1987 Sep;169(9):4092–4098. doi: 10.1128/jb.169.9.4092-4098.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Conformation change of cytochrome c. I. Ferrocytochrome c structure refined at 1.5 A resolution. J Mol Biol. 1981 Nov 25;153(1):79–94. doi: 10.1016/0022-2836(81)90528-3. [DOI] [PubMed] [Google Scholar]
- Tsuboi A., Tsukagoshi N., Udaka S. Reassembly in vitro of hexagonal surface arrays in a protein-producing bacterium, Bacillus brevis 47. J Bacteriol. 1982 Sep;151(3):1485–1497. doi: 10.1128/jb.151.3.1485-1497.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildhaber I., Santarius U., Baumeister W. Three-dimensional structure of the surface protein of Desulfurococcus mobilis. J Bacteriol. 1987 Dec;169(12):5563–5568. doi: 10.1128/jb.169.12.5563-5568.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Olsen G. J. Archaebacterial phylogeny: perspectives on the urkingdoms. Syst Appl Microbiol. 1986;7:161–177. doi: 10.1016/s0723-2020(86)80001-7. [DOI] [PubMed] [Google Scholar]