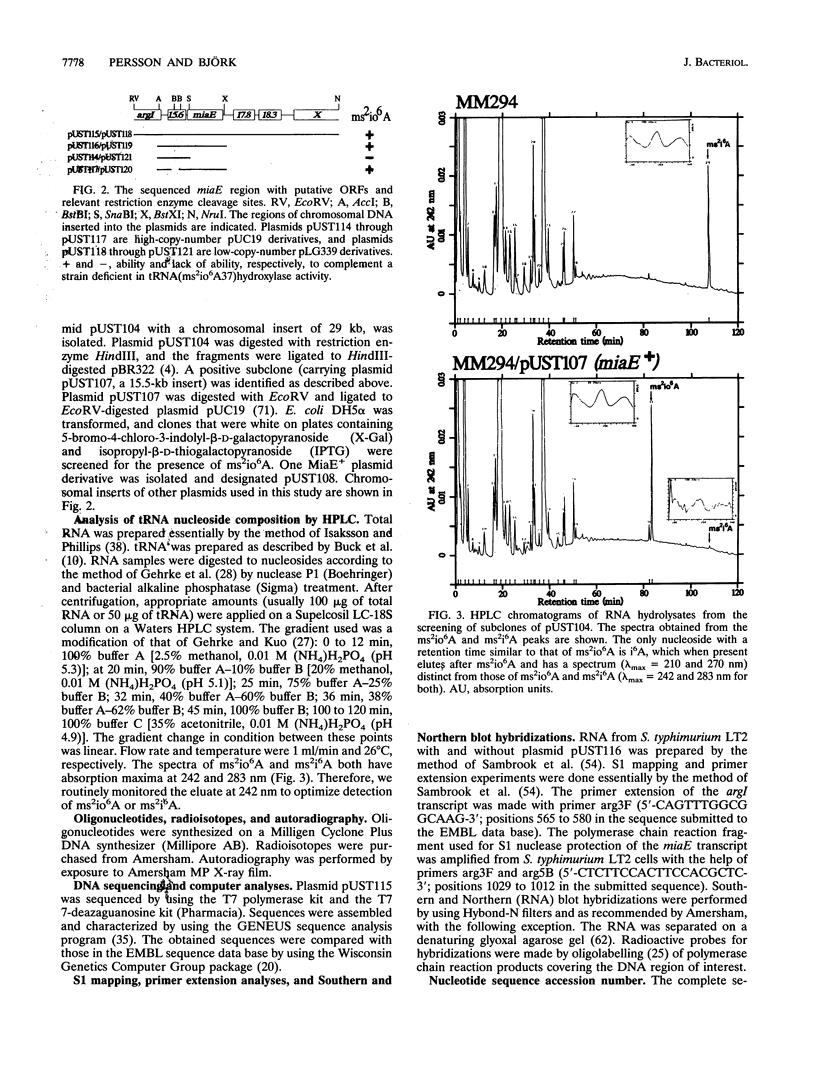
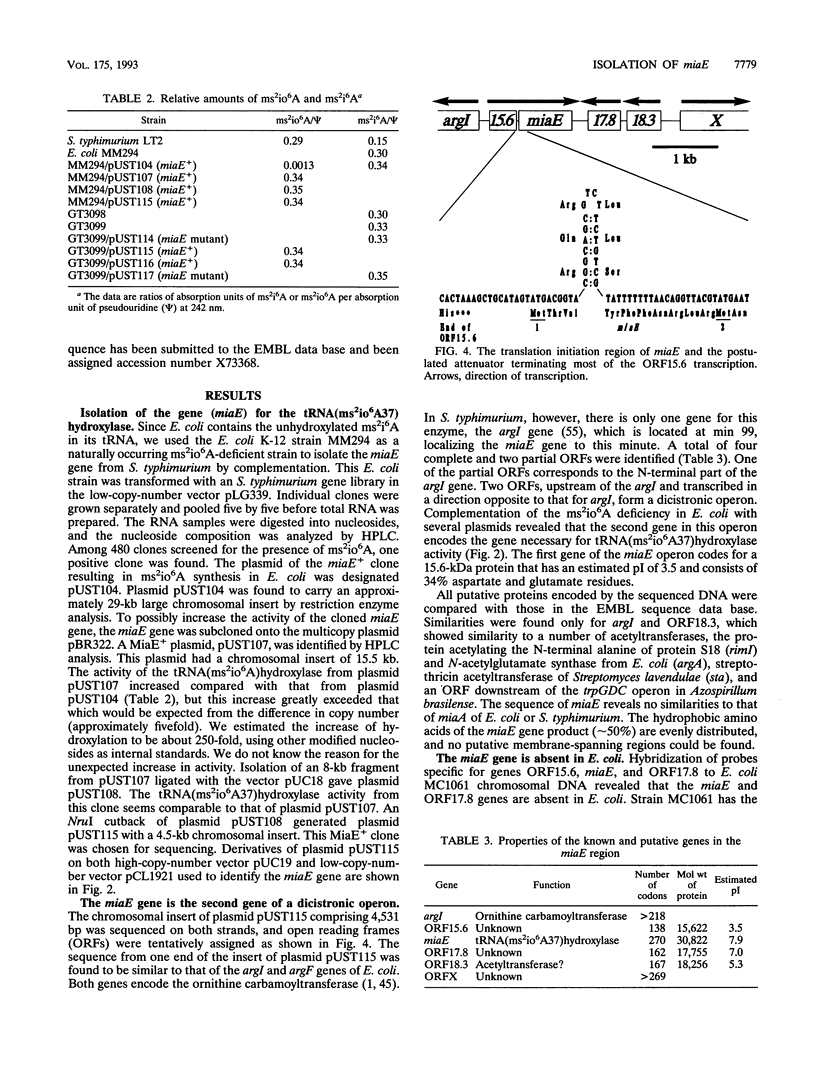
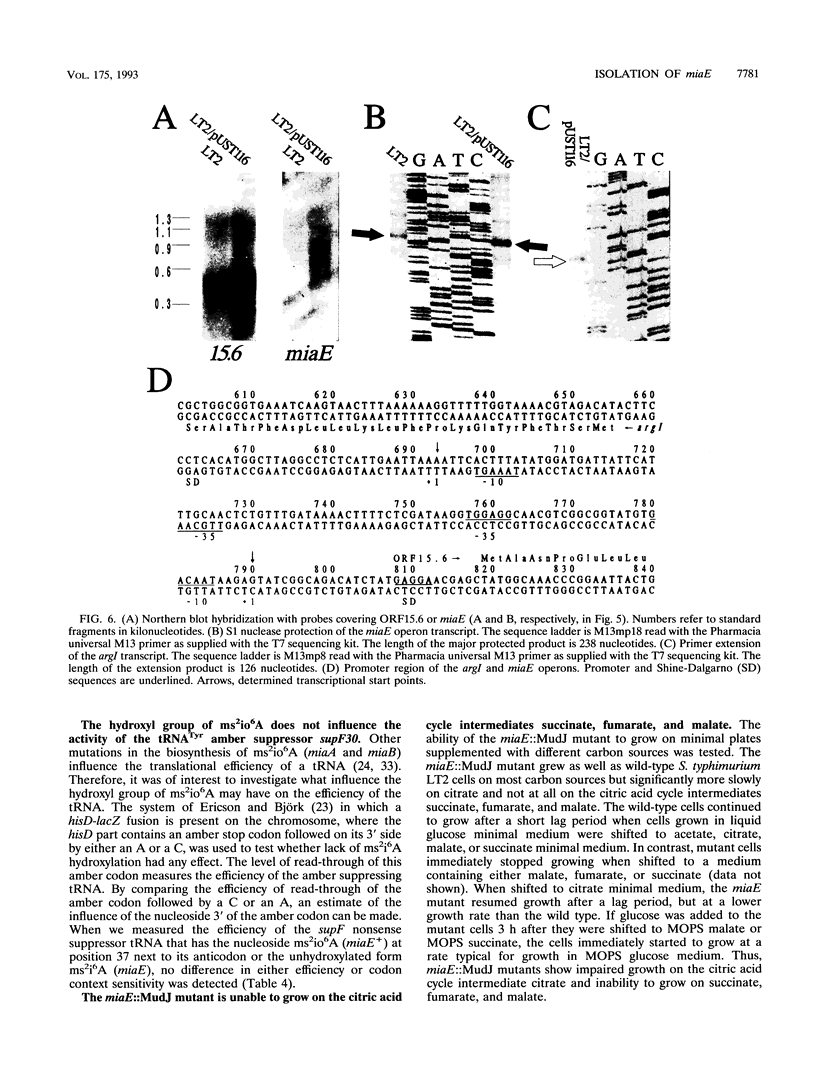
Abstract
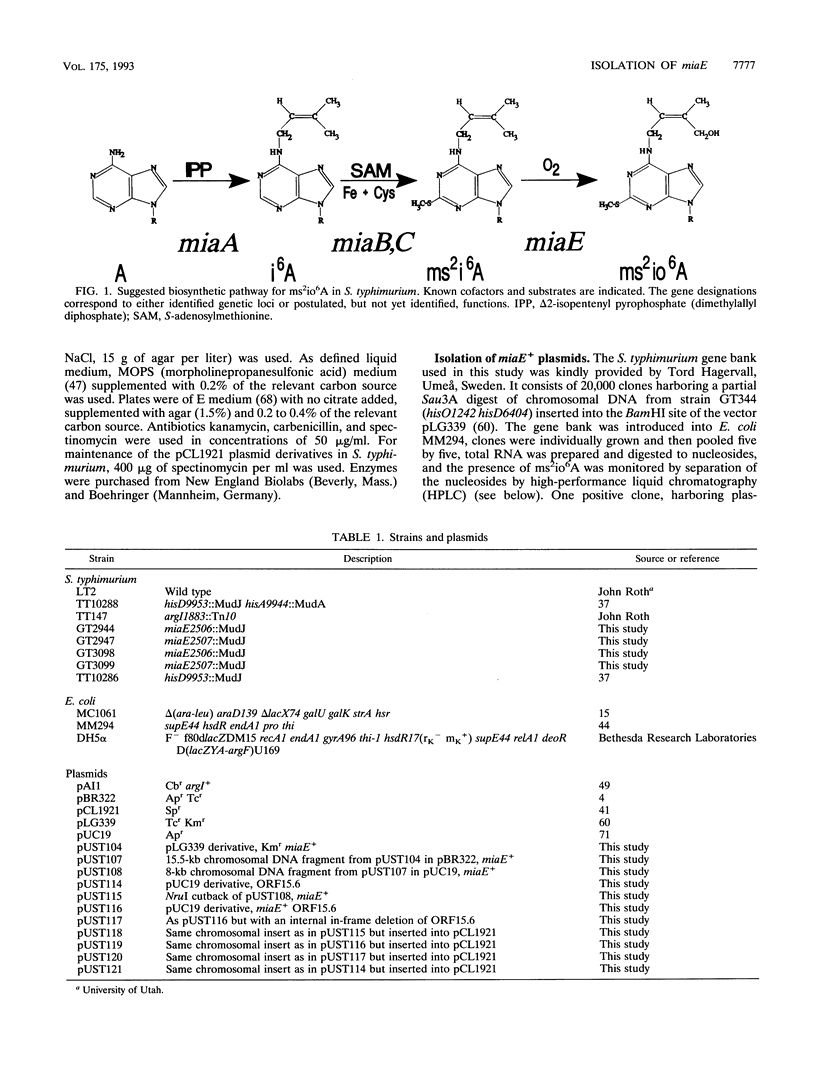
The modified nucleoside 2-methylthio-N-6-isopentenyl adenosine (ms2i6A) is present at position 37 (3' of the anticodon) of tRNAs that read codons beginning with U except tRNA(I,V Ser) in Escherichia coli. Salmonella typhimurium 2-methylthio-cis-ribozeatin (ms2io6A) is found in tRNA, probably in the corresponding species that have ms2i6A in E. coli. The gene (miaE) for the tRNA(ms2io6A)hydroxylase of S. typhimurium was isolated by complementation in E. coli. The miaE gene was localized close to the argI gene at min 99 of the S. typhimurium chromosomal map. Its DNA sequence and transcription pattern together with complementation studies revealed that the miaE gene is the second gene of a dicistronic operon. Southern blot analysis showed that the miaE gene is absent in E. coli, a finding consistent with the absence of the hydroxylated derivative of ms2i6A in this species. Mutants of S. typhimurium which have MudJ inserted in the miaE gene and which, consequently, are blocked in the ms2i6A hydroxylation reaction were isolated. Unexpectedly, such mutants cannot utilize the citric acid cycle intermediates malate, fumarate, and succinate as carbon sources.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencini D. A., Houghton J. E., Hoover T. A., Foltermann K. F., Wild J. R., O'Donovan G. A. The DNA sequence of argI from Escherichia coli K12. Nucleic Acids Res. 1983 Dec 10;11(23):8509–8518. doi: 10.1093/nar/11.23.8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum P. H., Ames B. N. Immunochemical identification of a tRNA-independent cytokinin-like compound in Salmonella typhimurium. Biochim Biophys Acta. 1989 Mar 1;1007(2):196–202. doi: 10.1016/0167-4781(89)90039-0. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bouadloun F., Srichaiyo T., Isaksson L. A., Björk G. R. Influence of modification next to the anticodon in tRNA on codon context sensitivity of translational suppression and accuracy. J Bacteriol. 1986 Jun;166(3):1022–1027. doi: 10.1128/jb.166.3.1022-1027.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boy E., Borne F., Patte J. C. Effect of mutations affecting lysyl-tRNAlys on the regulation of lysine biosynthesis in Escherichia coli. Mol Gen Genet. 1978 Feb 7;159(1):33–38. doi: 10.1007/BF00401745. [DOI] [PubMed] [Google Scholar]
- Bresalier R. S., Rizzino A. A., Freundlich M. Reduced maximal levels of derepression of the isoleucine-valine and leucine enzymes in hisT mutants of Salmonella typhimurium. Nature. 1975 Jan 24;253(5489):279–280. doi: 10.1038/253279a0. [DOI] [PubMed] [Google Scholar]
- Buck M., Ames B. N. A modified nucleotide in tRNA as a possible regulator of aerobiosis: synthesis of cis-2-methyl-thioribosylzeatin in the tRNA of Salmonella. Cell. 1984 Feb;36(2):523–531. doi: 10.1016/0092-8674(84)90245-9. [DOI] [PubMed] [Google Scholar]
- Buck M., Connick M., Ames B. N. Complete analysis of tRNA-modified nucleosides by high-performance liquid chromatography: the 29 modified nucleosides of Salmonella typhimurium and Escherichia coli tRNA. Anal Biochem. 1983 Feb 15;129(1):1–13. doi: 10.1016/0003-2697(83)90044-1. [DOI] [PubMed] [Google Scholar]
- Buck M., Griffiths E. Iron mediated methylthiolation of tRNA as a regulator of operon expression in Escherichia coli. Nucleic Acids Res. 1982 Apr 24;10(8):2609–2624. doi: 10.1093/nar/10.8.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M., Griffiths E. Regulation of aromatic amino acid transport by tRNA: role of 2-methylthio-N6-(delta2-isopentenyl)-adenosine. Nucleic Acids Res. 1981 Jan 24;9(2):401–414. doi: 10.1093/nar/9.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M., McCloskey J. A., Basile B., Ames B. N. cis 2-Methylthio-ribosylzeatin (ms2io6A) is present in the transfer RNA of Salmonella typhimurium, but not Escherichia coli. Nucleic Acids Res. 1982 Sep 25;10(18):5649–5662. doi: 10.1093/nar/10.18.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillet J., Droogmans L. Molecular cloning of the Escherichia coli miaA gene involved in the formation of delta 2-isopentenyl adenosine in tRNA. J Bacteriol. 1988 Sep;170(9):4147–4152. doi: 10.1128/jb.170.9.4147-4152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Cherayil J. D., Lipsett M. N. Zeatin ribonucleosides in the transfer ribonucleic acid of Rhizobium leguminosarum, Agrobacterium tumefaciens, Corynebacterium fascians, and Erwinia amylovora. J Bacteriol. 1977 Sep;131(3):741–744. doi: 10.1128/jb.131.3.741-744.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly D. M., Winkler M. E. Genetic and physiological relationships among the miaA gene, 2-methylthio-N6-(delta 2-isopentenyl)-adenosine tRNA modification, and spontaneous mutagenesis in Escherichia coli K-12. J Bacteriol. 1989 Jun;171(6):3233–3246. doi: 10.1128/jb.171.6.3233-3246.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly D. M., Winkler M. E. Structure of Escherichia coli K-12 miaA and characterization of the mutator phenotype caused by miaA insertion mutations. J Bacteriol. 1991 Mar;173(5):1711–1721. doi: 10.1128/jb.173.5.1711-1721.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese R., Landsberg R., Haar R. A., Umbarger H. E., Ames B. N. Pleiotropy of hisT mutants blocked in pseudouridine synthesis in tRNA: leucine and isoleucine-valine operons. Proc Natl Acad Sci U S A. 1974 May;71(5):1857–1861. doi: 10.1073/pnas.71.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz I., Pedersen S., Kurland C. G. Effects of miaA on translation and growth rates. Mol Gen Genet. 1987 Jul;208(3):373–376. doi: 10.1007/BF00328126. [DOI] [PubMed] [Google Scholar]
- Ericson J. U., Björk G. R. Pleiotropic effects induced by modification deficiency next to the anticodon of tRNA from Salmonella typhimurium LT2. J Bacteriol. 1986 Jun;166(3):1013–1021. doi: 10.1128/jb.166.3.1013-1021.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J. U., Björk G. R. tRNA anticodons with the modified nucleoside 2-methylthio-N6-(4-hydroxyisopentenyl)adenosine distinguish between bases 3' of the codon. J Mol Biol. 1991 Apr 5;218(3):509–516. doi: 10.1016/0022-2836(91)90697-5. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Russell R. L. Role modifications in tyrosine transfer RNA: a modified base affecting ribosome binding. J Mol Biol. 1969 Jan 14;39(1):145–157. doi: 10.1016/0022-2836(69)90339-8. [DOI] [PubMed] [Google Scholar]
- Gehrke C. W., Kuo K. C., McCune R. A., Gerhardt K. O., Agris P. F. Quantitative enzymatic hydrolysis of tRNAs: reversed-phase high-performance liquid chromatography of tRNA nucleosides. J Chromatogr. 1982 Jul 9;230(2):297–308. [PubMed] [Google Scholar]
- Gollnick P., Yanofsky C. tRNA(Trp) translation of leader peptide codon 12 and other factors that regulate expression of the tryptophanase operon. J Bacteriol. 1990 Jun;172(6):3100–3107. doi: 10.1128/jb.172.6.3100-3107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar J., Pittard J. Regulation of phenylalanine biosynthesis in Escherichia coli K-12: control of transcription of the pheA operon. J Bacteriol. 1982 Jun;150(3):1130–1137. doi: 10.1128/jb.150.3.1130-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths E., Humphreys J. Alterations in tRNAs containing 2-methylthio-N6-(delta2-isopentenyl)-adenosine during growth of enteropathogenic Escherichia coli in the presence of iron-binding proteins. Eur J Biochem. 1978 Jan 16;82(2):503–513. doi: 10.1111/j.1432-1033.1978.tb12044.x. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Nicoghosian K., Haumont E., Söll D., Cedergren R. Nucleotide sequences of two serine tRNAs with a GGA anticodon: the structure-function relationships in the serine family of E. coli tRNAs. Nucleic Acids Res. 1985 Aug 12;13(15):5697–5706. doi: 10.1093/nar/13.15.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagervall T. G., Ericson J. U., Esberg K. B., Li J. N., Björk G. R. Role of tRNA modification in translational fidelity. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):263–266. doi: 10.1016/0167-4781(90)90178-5. [DOI] [PubMed] [Google Scholar]
- Hall R. H., Csonka L., David H., McLennan B. Cytokinins in the soluble RNA of plant tissues. Science. 1967 Apr 7;156(3771):69–71. doi: 10.1126/science.156.3771.69. [DOI] [PubMed] [Google Scholar]
- Harr R., Fällman P., Häggström M., Wahlström L., Gustafsson P. GENEUS, a computer system for DNA and protein sequence analysis containing an information retrieval system for the EMBL data library. Nucleic Acids Res. 1986 Jan 10;14(1):273–284. doi: 10.1093/nar/14.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D. D., Wang W. Y., Gough S. P., Kannangara C. G. delta-Aminolevulinic acid-synthesizing enzymes need an RNA moiety for activity. Science. 1984 Sep 28;225(4669):1482–1484. doi: 10.1126/science.6206568. [DOI] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Transitory cis complementation: a method for providing transposition functions to defective transposons. Genetics. 1988 May;119(1):9–12. doi: 10.1093/genetics/119.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson L. A., Phillips J. H. Studies on microbial RNA. V. A comparison of the in vivo methylated components of ribosomal RNA from Escherichia coli and Saccharomyces cerevisiae. Biochim Biophys Acta. 1968 Jan 29;155(1):63–71. [PubMed] [Google Scholar]
- Janzer J. J., Raney J. P., McLennan B. D. The transfer RNA of certain Enterobacteriacae contain 2-methylthiozeatin riboside (ms2io6A) an isopentenyl adenosine derivative. Nucleic Acids Res. 1982 Sep 25;10(18):5663–5672. doi: 10.1093/nar/10.18.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A., Elseviers D., Gorini L. Isolation and characterization of lambda transducing bacteriophages for argF, argI and adjacent genes. J Bacteriol. 1975 May;122(2):727–742. doi: 10.1128/jb.122.2.727-742.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner C. G., Inouye M. Low copy number plasmids for regulated low-level expression of cloned genes in Escherichia coli with blue/white insert screening capability. Nucleic Acids Res. 1990 Aug 11;18(15):4631–4631. doi: 10.1093/nar/18.15.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDEL L. R., BOREK E. Variability in the structure of ribonucleic acid. Biochem Biophys Res Commun. 1961 Jan 25;4:14–18. doi: 10.1016/0006-291x(61)90246-7. [DOI] [PubMed] [Google Scholar]
- McCray J. W., Jr, Herrmann K. M. Derepression of certain aromatic amino acid biosynthetic enzymes of Escherichia coli K-12 by growth in Fe3+-deficient medium. J Bacteriol. 1976 Feb;125(2):608–615. doi: 10.1128/jb.125.2.608-615.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Moore S. K., Garvin R. T., James E. Nucleotide sequence of the argF regulatory region of Escherichia coli K-12. Gene. 1981 Dec;16(1-3):119–132. doi: 10.1016/0378-1119(81)90068-8. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nègre D., Cortay J. C., Donini P., Cozzone A. J. Relationship between guanosine tetraphosphate and accuracy of translation in Salmonella typhimurium. Biochemistry. 1989 Feb 21;28(4):1814–1819. doi: 10.1021/bi00430a058. [DOI] [PubMed] [Google Scholar]
- Petrullo L. A., Elseviers D. Effect of a 2-methylthio-N6-isopentenyladenosine deficiency on peptidyl-tRNA release in Escherichia coli. J Bacteriol. 1986 Feb;165(2):608–611. doi: 10.1128/jb.165.2.608-611.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Cunin R., Van Vliet F., Charlier D., Crabeel M., Ota Y., Glansdorff N. Homologous control sites and DNA transcription starts in the related argF and argI genes of Escherichia coli K12. EMBO J. 1982;1(7):853–857. doi: 10.1002/j.1460-2075.1982.tb01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzino A. A., Bresalier R. S., Freundlich M. Derepressed levels of the isoleucine-valine and leucine enzymes in his T 1504, a strain of Salmonella typhimurium with altered leucine transfer ribonucleic acid. J Bacteriol. 1974 Feb;117(2):449–455. doi: 10.1128/jb.117.2.449-455.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Gefter M. L. An iron-dependent modification of several transfer RNA species in Escherichia coli. J Mol Biol. 1969 Dec 28;46(3):581–584. doi: 10.1016/0022-2836(69)90197-1. [DOI] [PubMed] [Google Scholar]
- Rosenfeld S. A., Brenchley J. E. Regulation of nitrogen utilization of hisT mutants of Salmonella typhimurium. J Bacteriol. 1980 Aug;143(2):801–808. doi: 10.1128/jb.143.2.801-808.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals J., Hsu R. Y., Lipsett M. N., Bremer H. Isolation of single-site Escherichia coli mutants deficient in thiamine and 4-thiouridine syntheses: identification of a nuvC mutant. J Bacteriol. 1982 Aug;151(2):899–904. doi: 10.1128/jb.151.2.899-904.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadaro A., Spena A., Santonastaso V., Conini P. Stringency without ppGpp accumulation. Nature. 1981 May 21;291(5812):256–258. doi: 10.1038/291256a0. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Meissner F., Moll J., Vorderwülbecke T. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1987;15 (Suppl):r53–188. doi: 10.1093/nar/15.suppl.r53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens J. C., Artz S. W., Ames B. N. Guanosine 5'-diphosphate 3'-diphosphate (ppGpp): positive effector for histidine operon transcription and general signal for amino-acid deficiency. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4389–4393. doi: 10.1073/pnas.72.11.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker N. G., Fairweather N. F., Spratt B. G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982 Jun;18(3):335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Cherayil J. D. Unique presence of 2-methylthio-ribosylzeatin in the transfer ribonucleic acid of the bacterium Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1974 Sep 23;60(2):665–672. doi: 10.1016/0006-291x(74)90292-7. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins G. M. The metabolic code. Science. 1975 Sep 5;189(4205):760–763. doi: 10.1126/science.169570. [DOI] [PubMed] [Google Scholar]
- Tsang T. H., Buck M., Ames B. N. Sequence specificity of tRNA-modifying enzymes. An analysis of 258 tRNA sequences. Biochim Biophys Acta. 1983 Nov 17;741(2):180–196. doi: 10.1016/0167-4781(83)90058-1. [DOI] [PubMed] [Google Scholar]
- Tsui H. C., Arps P. J., Connolly D. M., Winkler M. E. Absence of hisT-mediated tRNA pseudouridylation results in a uracil requirement that interferes with Escherichia coli K-12 cell division. J Bacteriol. 1991 Nov;173(22):7395–7400. doi: 10.1128/jb.173.22.7395-7400.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Neill R. J., Landsberg R., Ames B. N. Pseudouridylation of tRNAs and its role in regulation in Salmonella typhimurium. J Biol Chem. 1979 Jun 25;254(12):5111–5119. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vacher J., Grosjean H., Houssier C., Buckingham R. H. The effect of point mutations affecting Escherichia coli tryptophan tRNA on anticodon-anticodon interactions and on UGA suppression. J Mol Biol. 1984 Aug 5;177(2):329–342. doi: 10.1016/0022-2836(84)90460-1. [DOI] [PubMed] [Google Scholar]
- Wang W. Y., Huang D. D., Stachon D., Gough S. P., Kannangara C. G. Purification, Characterization, and Fractionation of the delta-Aminolevulinic Acid Synthesizing Enzymes from Light-Grown Chlamydomonas reinhardtii Cells. Plant Physiol. 1984 Mar;74(3):569–575. doi: 10.1104/pp.74.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein F. O., Stent G. S. Physiologically induced changes in the property of phenylalanine tRNA in Escherichia coli. J Mol Biol. 1968 Nov 28;38(1):25–40. doi: 10.1016/0022-2836(68)90126-5. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Mutations affecting tRNATrp and its charging and their effect on regulation of transcription termination at the attenuator of the tryptophan operon. J Mol Biol. 1977 Jul 15;113(4):663–677. doi: 10.1016/0022-2836(77)90229-7. [DOI] [PubMed] [Google Scholar]
- Zhang J., Deutscher M. P. A uridine-rich sequence required for translation of prokaryotic mRNA. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2605–2609. doi: 10.1073/pnas.89.7.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Brown K., Killingly D., Yanofsky C. Nucleotide sequence of the leader region of the phenylalanine operon of Escherichia coli. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4271–4275. doi: 10.1073/pnas.75.9.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]




