Abstract
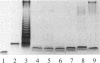
By mutational analysis it was found that a 3.9-kb SmaI-XhoII DNA fragment of Xanthomonas campestris pv. campestris is involved in lipopolysaccharide (LPS) biosynthesis. LPS samples isolated from different mutants carrying mutations in the 3.9-kb SmaI-XhoII DNA fragment exhibited banding patterns in silver-stained sodium dodecyl sulfate-polyacrylamide gels markedly different from that of the wild-type LPS. Moreover, comparison of the monosaccharide composition obtained by high-performance anion-exchange chromatography with pulsed amperometric detection of LPS purified from wild-type Xanthomonas campestris pv. campestris B100 and from mutants with mutations in the 3.9-kb SmaI-XhoII DNA fragment revealed a lack of rhamnose moieties in the mutant LPS. Sequence analysis of this DNA fragment revealed four open reading frames (ORFs), designated ORF302, ORF183, ORF295, and ORF351. The deduced amino acid sequences of these ORFs showed a high degree of homology to the deduced amino acid sequences of the rfbC, rfbD, rfbA, and rfbB genes of Salmonella typhimurium LT2, which have been shown to encode a set of enzymes responsible for conversion of glucose 1-phosphate to dTDP-rhamnose.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W., Rump A., Klipp W., Priefer U. B., Pühler A. Nucleotide sequence of a 24,206-base-pair DNA fragment carrying the entire nitrogen fixation gene cluster of Klebsiella pneumoniae. J Mol Biol. 1988 Oct 5;203(3):715–738. doi: 10.1016/0022-2836(88)90205-7. [DOI] [PubMed] [Google Scholar]
- BERNSTEIN R. L., ROBBINS P. W. CONTROL ASPECTS OF URIDINE 5'-DIPHOSPHATE GLUCOSE AND THYMIDINE 5'-DIPHOSPHATE GLUCOSE SYNTHESIS BY MICROBIAL ENZYMES. J Biol Chem. 1965 Jan;240:391–397. [PubMed] [Google Scholar]
- Cava J. R., Elias P. M., Turowski D. A., Noel K. D. Rhizobium leguminosarum CFN42 genetic regions encoding lipopolysaccharide structures essential for complete nodule development on bean plants. J Bacteriol. 1989 Jan;171(1):8–15. doi: 10.1128/jb.171.1.8-15.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Electrophoretic separation of lipopolysaccharide monomers differing in polysaccharide length. Methods Enzymol. 1987;138:267–275. doi: 10.1016/0076-6879(87)38022-x. [DOI] [PubMed] [Google Scholar]
- Harding N. E., Cleary J. M., Cabañas D. K., Rosen I. G., Kang K. S. Genetic and physical analyses of a cluster of genes essential for xanthan gum biosynthesis in Xanthomonas campestris. J Bacteriol. 1987 Jun;169(6):2854–2861. doi: 10.1128/jb.169.6.2854-2861.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick C. A., Sequeira L. Lipopolysaccharide-Defective Mutants of the Wilt Pathogen Pseudomonas solanacearum. Appl Environ Microbiol. 1984 Jul;48(1):94–101. doi: 10.1128/aem.48.1.94-101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman J., Ashwell G. Isolation of a bacterial lipopolysaccharide from Xanthomonas campestris containing 3-acetamido-3,6-dideoxy-D-galactose and D-rhamnose. J Biol Chem. 1966 Mar 25;241(6):1424–1428. [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hötte B., Rath-Arnold I., Pühler A., Simon R. Cloning and analysis of a 35.3-kilobase DNA region involved in exopolysaccharide production by Xanthomonas campestris pv. campestris. J Bacteriol. 1990 May;172(5):2804–2807. doi: 10.1128/jb.172.5.2804-2807.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X. M., Neal B., Santiago F., Lee S. J., Romana L. K., Reeves P. R. Structure and sequence of the rfb (O antigen) gene cluster of Salmonella serovar typhimurium (strain LT2). Mol Microbiol. 1991 Mar;5(3):695–713. doi: 10.1111/j.1365-2958.1991.tb00741.x. [DOI] [PubMed] [Google Scholar]
- Komeda Y., Icho T., Iino T. Effects of galU mutation on flagellar formation in Escherichia coli. J Bacteriol. 1977 Feb;129(2):908–915. doi: 10.1128/jb.129.2.908-915.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köplin R., Arnold W., Hötte B., Simon R., Wang G., Pühler A. Genetics of xanthan production in Xanthomonas campestris: the xanA and xanB genes are involved in UDP-glucose and GDP-mannose biosynthesis. J Bacteriol. 1992 Jan;174(1):191–199. doi: 10.1128/jb.174.1.191-199.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesse A. J., Campagnari A. A., Bittner W. E., Apicella M. A. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods. 1990 Jan 24;126(1):109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Marumo K., Lindqvist L., Verma N., Weintraub A., Reeves P. R., Lindberg A. A. Enzymatic synthesis and isolation of thymidine diphosphate-6-deoxy-D-xylo-4-hexulose and thymidine diphosphate-L-rhamnose. Production using cloned gene products and separation by HPLC. Eur J Biochem. 1992 Mar 1;204(2):539–545. doi: 10.1111/j.1432-1033.1992.tb16665.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Reeves P. Evolution of Salmonella O antigen variation by interspecific gene transfer on a large scale. Trends Genet. 1993 Jan;9(1):17–22. doi: 10.1016/0168-9525(93)90067-R. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit G., Kijne J. W., Lugtenberg B. J. Roles of flagella, lipopolysaccharide, and a Ca2+-dependent cell surface protein in attachment of Rhizobium leguminosarum biovar viciae to pea root hair tips. J Bacteriol. 1989 Jan;171(1):569–572. doi: 10.1128/jb.171.1.569-572.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. The current status and portability of our sequence handling software. Nucleic Acids Res. 1986 Jan 10;14(1):217–231. doi: 10.1093/nar/14.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W., Kennedy A. F. Comparison of bacterial lipopolysaccharides by high-performance liquid chromatography. Appl Environ Microbiol. 1986 Oct;52(4):948–950. doi: 10.1128/aem.52.4.948-950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne L., Tansey L., Pollock T. J. Clustering of mutations blocking synthesis of xanthan gum by Xanthomonas campestris. J Bacteriol. 1987 Aug;169(8):3593–3600. doi: 10.1128/jb.169.8.3593-3600.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Volk W. A. Cell wall lipopolysaccharides from xanthomonas species. J Bacteriol. 1966 Jan;91(1):39–42. doi: 10.1128/jb.91.1.39-42.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga R. K., Hol W. G. Predicted nucleotide-binding properties of p21 protein and its cancer-associated variant. Nature. 1983 Apr 28;302(5911):842–844. doi: 10.1038/302842a0. [DOI] [PubMed] [Google Scholar]
- Zarkowsky H., Glaser L. The mechanism of 6-deoxyhexose synthesis. 3. Purification of deosythymidine diphosphate-glucose oxidoreductase. J Biol Chem. 1969 Sep 10;244(17):4750–4756. [PubMed] [Google Scholar]