Abstract
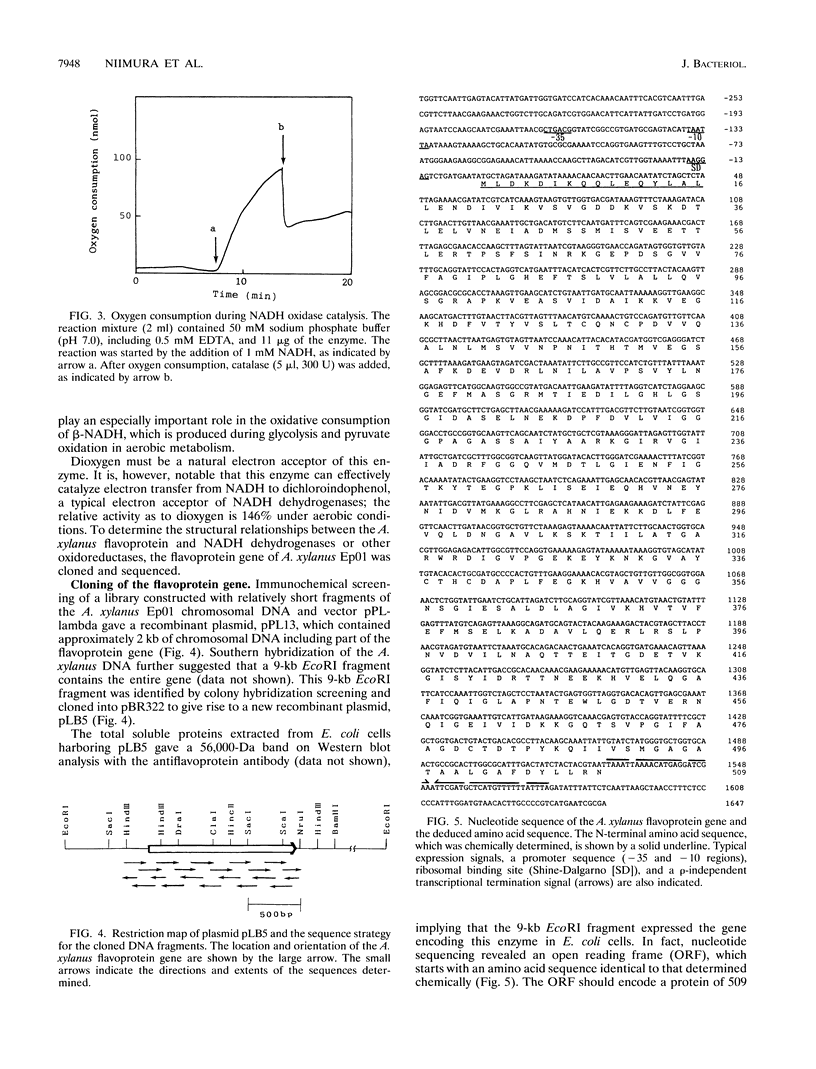
Amphibacillus xylanus Ep01, a facultative anaerobe we recently isolated, shows rapid aerobic growth even though it lacks a respiratory pathway. Thus, the oxidative consumption of NADH, produced during glycolysis and pyruvate oxidation, should be especially important for maintenance of intracellular redox balance in this bacterium. We purified a flavoprotein functional as NADH oxidase from aerobically growing A. xylanus Ep01. The A. xylanus enzyme is a homotetramer composed of a subunit (M(r) 56,000) containing 1 mol of flavin adenine dinucleotide. This enzyme catalyzes the reduction of oxygen to hydrogen peroxide with beta-NADH as the preferred electron donor and exhibits no activity with NADPH. The flavoprotein gene of A. xylanus Ep01 was cloned by using a specific antibody. The amino acid sequence of 509 residues, deduced from the nucleotide sequence, showed 51.2 and 72.5% identities to the amino acid sequences of alkyl hydroperoxide reductase from Salmonella typhimurium and NADH dehydrogenase from alkalophilic Bacillus sp. strain YN-1, respectively. Bacillus spp. have a respiratory chain and grow well under aerobic conditions. In contrast, Amphibacillus spp., having no respiratory chain, grow equally well under both aerobic and anaerobic conditions, which distinguishes these two genera. Salmonella spp., which are gram-negative bacteria, are taxonomically distant from gram-positive bacteria such as Bacillus spp. and Amphibacillus spp. The above findings, however, suggest that the flavoprotein functional as NADH oxidase, the alkyl hydroperoxide reductase, and the NADH dehydrogenase diverged recently, with only small changes leading to their functional differences.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S. A., Claiborne A. The streptococcal flavoprotein NADH oxidase. I. Evidence linking NADH oxidase and NADH peroxidase cysteinyl redox centers. J Biol Chem. 1989 Nov 25;264(33):19856–19863. [PubMed] [Google Scholar]
- Ahmed S. A., Claiborne A. The streptococcal flavoprotein NADH oxidase. II. Interactions of pyridine nucleotides with reduced and oxidized enzyme forms. J Biol Chem. 1989 Nov 25;264(33):19863–19870. [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Cocco D., Rinaldi A., Savini I., Cooper J. M., Bannister J. V. NADH oxidase from the extreme thermophile Thermus aquaticus YT-1. Purification and characterisation. Eur J Biochem. 1988 Jun 1;174(2):267–271. doi: 10.1111/j.1432-1033.1988.tb14093.x. [DOI] [PubMed] [Google Scholar]
- Eggink G., Engel H., Vriend G., Terpstra P., Witholt B. Rubredoxin reductase of Pseudomonas oleovorans. Structural relationship to other flavoprotein oxidoreductases based on one NAD and two FAD fingerprints. J Mol Biol. 1990 Mar 5;212(1):135–142. doi: 10.1016/0022-2836(90)90310-I. [DOI] [PubMed] [Google Scholar]
- Götz F., Sedewitz B., Elstner E. F. Oxygen utilization by Lactobacillus plantarum. I. Oxygen consuming reactions. Arch Microbiol. 1980 Apr;125(3):209–214. doi: 10.1007/BF00446878. [DOI] [PubMed] [Google Scholar]
- Jacobson F. S., Morgan R. W., Christman M. F., Ames B. N. An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage. Purification and properties. J Biol Chem. 1989 Jan 25;264(3):1488–1496. [PubMed] [Google Scholar]
- Koike K., Kobayashi T., Ito S., Saitoh M. Purification and characterization of NADH oxidase from a strain of Leuconostoc mesenteroides. J Biochem. 1985 May;97(5):1279–1288. doi: 10.1093/oxfordjournals.jbchem.a135179. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Brien R. W., Morris J. G. Oxygen and the growth and metabolism of Clostridium acetobutylicum. J Gen Microbiol. 1971 Nov;68(3):307–318. doi: 10.1099/00221287-68-3-307. [DOI] [PubMed] [Google Scholar]
- Park H. J., Kreutzer R., Reiser C. O., Sprinzl M. Molecular cloning and nucleotide sequence of the gene encoding a H2O2-forming NADH oxidase from the extreme thermophilic Thermus thermophilus HB8 and its expression in Escherichia coli. Eur J Biochem. 1992 May 1;205(3):875–879. doi: 10.1111/j.1432-1033.1992.tb16852.x. [DOI] [PubMed] [Google Scholar]
- Park H. J., Reiser C. O., Kondruweit S., Erdmann H., Schmid R. D., Sprinzl M. Purification and characterization of a NADH oxidase from the thermophile Thermus thermophilus HB8. Eur J Biochem. 1992 May 1;205(3):881–885. doi: 10.1111/j.1432-1033.1992.tb16853.x. [DOI] [PubMed] [Google Scholar]
- Poole L. B., Claiborne A. Interactions of pyridine nucleotides with redox forms of the flavin-containing NADH peroxidase from Streptococcus faecalis. J Biol Chem. 1986 Nov 5;261(31):14525–14533. [PubMed] [Google Scholar]
- Reinards R., Kubicki J., Ohlenbusch H. D. Purification and characterization of NADH oxidase from membranes of Acholeplasma laidlawii, a copper-containing iron-sulfur flavoprotein. Eur J Biochem. 1981 Nov;120(2):329–337. doi: 10.1111/j.1432-1033.1981.tb05708.x. [DOI] [PubMed] [Google Scholar]
- Ritchey T. W., Seely H. W., Jr Distribution of cytochrome-like respiration in streptococci. J Gen Microbiol. 1976 Apr;93(2):195–203. doi: 10.1099/00221287-93-2-195. [DOI] [PubMed] [Google Scholar]
- Ross R. P., Claiborne A. Molecular cloning and analysis of the gene encoding the NADH oxidase from Streptococcus faecalis 10C1. Comparison with NADH peroxidase and the flavoprotein disulfide reductases. J Mol Biol. 1992 Oct 5;227(3):658–671. doi: 10.1016/0022-2836(92)90215-6. [DOI] [PubMed] [Google Scholar]
- Russel M., Model P. Sequence of thioredoxin reductase from Escherichia coli. Relationship to other flavoprotein disulfide oxidoreductases. J Biol Chem. 1988 Jun 25;263(18):9015–9019. [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Saeki Y., Nozaki M., Matsumoto K. Purification and properties of NADH oxidase from Bacillus megaterium. J Biochem. 1985 Dec;98(6):1433–1440. doi: 10.1093/oxfordjournals.jbchem.a135411. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. L., Stöcklein W., Danzer J., Kirch P., Limbach B. Isolation and properties of an H2O-forming NADH oxidase from Streptococcus faecalis. Eur J Biochem. 1986 Apr 1;156(1):149–155. doi: 10.1111/j.1432-1033.1986.tb09560.x. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Storz G., Brodsky M. H., Lai A., Ames B. N. Alkyl hydroperoxide reductase from Salmonella typhimurium. Sequence and homology to thioredoxin reductase and other flavoprotein disulfide oxidoreductases. J Biol Chem. 1990 Jun 25;265(18):10535–10540. [PubMed] [Google Scholar]
- Wierenga R. K., Terpstra P., Hol W. G. Prediction of the occurrence of the ADP-binding beta alpha beta-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986 Jan 5;187(1):101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- Xu X. M., Koyama N., Cui M., Yamagishi A., Nosoh Y., Oshima T. Nucleotide sequence of the gene encoding NADH dehydrogenase from an alkalophile, Bacillus sp. strain YN-1. J Biochem. 1991 May;109(5):678–683. doi: 10.1093/oxfordjournals.jbchem.a123440. [DOI] [PubMed] [Google Scholar]