Abstract
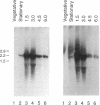
The two predominant polypeptides of the Bacillus thuringiensis subsp. thompsoni crystal are encoded by the cry40 and cry34 genes. These crystal protein genes are located in an operon. Western analysis (immunoblotting) demonstrated that the operon promoter activity was located in the region upstream of the cry40 gene. The Cry34 protein was expressed only when the upstream promoter region was present. The crystal protein genes are the only cistrons in the operon, and they are expressed during sporulation, with the highest transcript levels detected early in sporulation (1.5 to 3 h after the onset of sporulation). Transcription initiates from two adjacent sites located 84 and 85 bases upstream of the cry40 translational start codon. The B. thuringiensis subsp. thompsoni crystal protein gene operon promoter aligned with other crystal protein gene promoters, which are activated from early to midsporulation and transcribed in vitro by the B. thuringiensis RNA polymerase E sigma 35.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I., Angelo N., Holt S. C. Regulation of extracellular protease production in Bacillus cereus T: characterization of mutants producing altered amounts of protease. J Bacteriol. 1971 Jun;106(3):1016–1025. doi: 10.1128/jb.106.3.1016-1025.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzard B. L., Schnepf H. E., Kronstad J. W. Expression of the cryIB crystal protein gene of Bacillus thuringiensis. Mol Gen Genet. 1991 Dec;231(1):59–64. doi: 10.1007/BF00293822. [DOI] [PubMed] [Google Scholar]
- Brown K. L., Whiteley H. R. Isolation of a Bacillus thuringiensis RNA polymerase capable of transcribing crystal protein genes. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4166–4170. doi: 10.1073/pnas.85.12.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. L., Whiteley H. R. Isolation of the second Bacillus thuringiensis RNA polymerase that transcribes from a crystal protein gene promoter. J Bacteriol. 1990 Dec;172(12):6682–6688. doi: 10.1128/jb.172.12.6682-6688.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. L., Whiteley H. R. Molecular characterization of two novel crystal protein genes from Bacillus thuringiensis subsp. thompsoni. J Bacteriol. 1992 Jan;174(2):549–557. doi: 10.1128/jb.174.2.549-557.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crickmore N., Ellar D. J. Involvement of a possible chaperonin in the efficient expression of a cloned CryIIA delta-endotoxin gene in Bacillus thuringiensis. Mol Microbiol. 1992 Jun;6(11):1533–1537. doi: 10.1111/j.1365-2958.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Doi R. H., Wang L. F. Multiple procaryotic ribonucleic acid polymerase sigma factors. Microbiol Rev. 1986 Sep;50(3):227–243. doi: 10.1128/mr.50.3.227-243.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Helmann J. D., Chamberlin M. J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- Höfte H., Whiteley H. R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989 Jun;53(2):242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Stragier P. Crisscross regulation of cell-type-specific gene expression during development in B. subtilis. Nature. 1992 Feb 13;355(6361):601–604. doi: 10.1038/355601a0. [DOI] [PubMed] [Google Scholar]
- Losick R., Youngman P., Piggot P. J. Genetics of endospore formation in Bacillus subtilis. Annu Rev Genet. 1986;20:625–669. doi: 10.1146/annurev.ge.20.120186.003205. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. R., Murray C. L., Rabinowitz J. C. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981 Nov 10;256(21):11283–11291. [PubMed] [Google Scholar]
- Meenakshi K., Jayaraman K. On the formation of crystal proteins during sporulation in Bacillus thuringiensis var. thuringiensis. Arch Microbiol. 1979 Jan 16;120(1):9–14. doi: 10.1007/BF00413265. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., Banner C. D., Haldenwang W. G., Losick R. Promoter for a developmentally regulated gene in Bacillus subtilis. Cell. 1981 Sep;25(3):783–791. doi: 10.1016/0092-8674(81)90186-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe E. S., Nickerson K. W., Bulla L. A., Jr, Aronson J. N. Separation of spores and parasporal crystals of Bacillus thuringiensis in gradients of certain x-ray contrasting agents. Appl Microbiol. 1975 Dec;30(6):1052–1053. doi: 10.1128/am.30.6.1052-1053.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Waalwijk C., Dullemans A. M., van Workum M. E., Visser B. Molecular cloning and the nucleotide sequence of the Mr 28 000 crystal protein gene of Bacillus thuringiensis subsp. israelensis. Nucleic Acids Res. 1985 Nov 25;13(22):8207–8217. doi: 10.1093/nar/13.22.8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. S., Ellar D. J. Bacillus thuringiensis var. israelensis delta-endotoxin. Nucleotide sequence and characterization of the transcripts in Bacillus thuringiensis and Escherichia coli. J Mol Biol. 1986 Sep 5;191(1):1–11. doi: 10.1016/0022-2836(86)90417-1. [DOI] [PubMed] [Google Scholar]
- Widner W. R., Whiteley H. R. Two highly related insecticidal crystal proteins of Bacillus thuringiensis subsp. kurstaki possess different host range specificities. J Bacteriol. 1989 Feb;171(2):965–974. doi: 10.1128/jb.171.2.965-974.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H. C., Schnepf H. E., Whiteley H. R. Transcriptional and translational start sites for the Bacillus thuringiensis crystal protein gene. J Biol Chem. 1983 Feb 10;258(3):1960–1967. [PubMed] [Google Scholar]
- Yoshisue H., Fukada T., Yoshida K., Sen K., Kurosawa S., Sakai H., Komano T. Transcriptional regulation of Bacillus thuringiensis subsp. israelensis mosquito larvicidal crystal protein gene cryIVA. J Bacteriol. 1993 May;175(9):2750–2753. doi: 10.1128/jb.175.9.2750-2753.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Yang Y., Jong A. Y. Mini-prep in ten minutes. Biotechniques. 1990 Feb;8(2):172–173. [PubMed] [Google Scholar]