Abstract
Many cell surface proteins are anchored to a membrane via a glycosylphosphatidylinositol (GPI), which is attached to the C termini in the endoplasmic reticulum. The inositol ring of phosphatidylinositol is acylated during biosynthesis of GPI. In mammalian cells, the acyl chain is added to glucosaminyl phosphatidylinositol at the third step in the GPI biosynthetic pathway and then is usually removed soon after the attachment of GPIs to proteins. The mechanisms and roles of the inositol acylation and deacylation have not been well clarified. Herein, we report derivation of human and Chinese hamster mutant cells defective in inositol acylation and the gene responsible, PIG-W. The surface expressions of GPI-anchored proteins on these mutant cells were greatly diminished, indicating the critical role of inositol acylation. PIG-W encodes a 504-amino acid protein expressed in the endoplasmic reticulum. PIG-W is most likely inositol acyltransferase itself because the tagged PIG-W affinity purified from transfected human cells had inositol acyltransferase activity and because both mutant cells were complemented with PIG-W homologs of Saccharomyces cerevisiae and Schizosaccharomyces pombe. The inositol acylation is not essential for the subsequent mannosylation, indicating that glucosaminyl phosphatidylinositol can flip from the cytoplasmic side to the luminal side of the endoplasmic reticulum.
INTRODUCTION
Glycosylphosphatidylinositol (GPI), a complex glycolipid, acts as a membrane anchor for many cell surface proteins (Tiede et al., 1999; McConville and Menon, 2000; Ikezawa, 2002). In mammalian cells, >100 different proteins are GPI anchored, including cell surface enzymes, receptors, and adhesion molecules. GPI-anchored proteins are enriched in “lipid rafts,” allowing their functional linkages to signal transduction systems (Simons and Toomre, 2000). GPI-anchored proteins are abundant in protozoan parasites, such as Trypanosoma and Plasmodium species (Ferguson, 1999; Gowda and Davidson, 1999). Biosynthesis of GPI is essential for the bloodstream form of T. brucei (Nagamune et al., 2000), which causes sleeping sickness and has been validated as a therapeutic target (Ferguson, 2000). For the development of trypanosome-specific GPI biosynthesis inhibitors, it is important to clarify the different characteristics of human and trypanosomal pathways.
GPI is synthesized in the endoplasmic reticulum (ER) from phosphatidylinositol (PI) through multiple steps and is attached to proteins bearing the C-terminal GPI attachment signal sequence (Ferguson, 1999; Kinoshita and Inoue, 2000). The first two precursors of GPI are synthesized by transfer of N-acetylglucosamine (GlcNAc) to the inositol of PI to generate N-acetylglucosaminyl-PI (GlcNAc-PI), followed by deN-acetylation to generate glucosaminyl-PI (GlcN-PI). At the third step, the mammalian and T. brucei GPI biosynthetic pathways become different. In mammalian cells, the inositol acylation, mostly palmitoylation, occurs at GlcN-PI to generate GlcN-acylPI, and this precedes the first mannosylation that generates Man-GlcN-acylPI (Urakaze et al., 1992; Stevens and Zhang, 1994; Doerrler et al., 1996; Smith et al., 1997). Once added, the inositol-linked acyl chain remains until the mature GPI anchor is attached to the protein. Usually, the acyl chain is removed from the GPI-anchored proteins by an inositol deacylase (Chen et al., 1998). In contrast, inositol acylation occurs only after the first mannosylation in T. brucei (that is, the inositol acylation occurs at Man-GlcN-PI to generate Man-GlcN-acylPI) (Guther and Ferguson, 1995; Smith et al., 1996b). The inositol acylation is required for the addition of the bridging phosphoethanolamine (P-EtN) to the third mannose (Guther and Ferguson, 1995).
The two initial reactions that generate GlcN-PI occur on the cytoplasmic side of the ER membrane (Vidugiriene and Menon, 1993; Watanabe et al., 1996; Nakamura et al., 1997), whereas PIG-M, which transfers the first mannose to GlcN-acylPI, functions on the luminal side of the ER (Maeda et al., 2001). Therefore, flipping should occur before the first mannosylation. Mutant cells defective in inositol acylation would be useful to determine whether inositol acylation is essential for flipping in mammalian cells and to further characterize the substrate specificities of the enzymes downstream in the biosynthetic pathway. Herein, we report the molecular cloning of rat PIG-W critical for inositol acylation and present evidence that inositol acylation is not essential for flipping of GPI but is important for the expression of GPI-anchored proteins on the cell surface.
MATERIALS AND METHODS
Cells and Culture
Human T lymphoma Molt4 cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS). Chinese hamster ovary (CHO) cells were cultured in Ham's F-12 medium supplemented with 10% FCS. Human B-lymphoblastoid JY25 cells (Hollander et al., 1988) and Thy-1-negative mutant cells of complementation class E derived from mouse lymphoma BW5147 (Hyman, 1988) were cultured in DMEM supplemented with 10% FCS. A 1D10 mutant cell was established by sorting CD59 negative cells from Molt4 cells (American Type Culture Collection, Manassas, VA), followed by limiting dilution. To establish CHOPA10.14 mutant cells, we screened for cells resistant to aerolysin, a bacterial toxin that binds to GPI-anchored proteins (Buckley, 1999), as described previously (Hong et al., 2002). Cells were stained for decay accelerating factor (DAF) and CD59 and analyzed in a FACScan cytometer (BD Biosciences, San Jose, CA) (Nakamura et al., 1997).
Plasmids
Rat PIG-W cDNA was subcloned into pME18Sf+, a mammalian expression vector, and pME-neo, pME18Sf+ bearing neo resistance gene, and the resulting plasmids were termed pME-rPIG-W and pME-neo-rPIG-W, respectively. To add FLAG tag to the N terminus of rat PIG-W, we replaced PIG-A cDNA of pME-FLAG-hPIG-A (Maeda et al., 1998) with rat PIG-W cDNA, followed by subcloning a XhoI-NotI fragment bearing FLAG-PIG-W into pME-neo. To add FLAG tag to the C termini of rat PIG-W, we replaced GPI8 cDNA of pME-hGPI8-FLAG with rat PIG-W, followed by subcloning a XhoI-NotI fragment bearing PIG-W-FLAG into pME-neo. These plasmids were termed pME-neo-FLAG-rPIG-W and pME-neo-rPIG-W-FLAG, respectively. To generate pMEEB-FLAG-rPIG-W-GST and pMEEB-FLAG-rPIG-W-FLAG-HAT, we replaced XhoI-MluI fragments bearing GPI8 cDNA of pME-hGPI8-GST (Ohishi et al., 2000) and pME-hGPI8-FLAG-HAT (Watanabe et al., 2000), respectively, with XhoI-XbaI fragment of pME-neo-FLAG-rPIG-W and XbaI-MluI fragment of pME-neo-rPIG-W-FLAG, respectively. We then prepared XhoI-Asp718 fragments bearing FLAG-rPIG-W-GST and FLAG-rPIG-W-FLAG-HAT and cloned them into XhoI-and Asp718-cut pME-EBO, respectively. pMEEB-hPIG-L has been previously reported (Nakamura et al., 1997).
Cloning PIG-W cDNA
Molt4 1D10 cells (108) were mixed with 300 μg each of the plasmids of the rat C6 glioma cDNA library bearing simian virus 40 (SV40) origin of replication (Nakamura et al., 1997) and pBSII-SV40T (ori-) for expression of SV40 large T protein in 4 ml of HEPES-buffered saline and were electroporated in 10 cuvettes at 250 V and 960 μF by using a Gene Pulser (Bio-Rad, Hercules, CA). The next day, dead cells were removed by centrifugation through Ficoll-Paque (Pharmacia, Peapack, NJ). Two days after transfection, cells were stained with fluorescein isothiocyanate-labeled anti-CD59 antibody (BD Biosciences PharMingen, San Diego, CA) and propidium iodide to gate out the dead cells, and 582 cells that restored CD59 expression were collected by a cell sorter (FACS-Vantage; BD Biosciences). Plasmid clones (103) were recovered from the cells. Pooled plasmids (90 μg) were retransfected with 270 μg of pBSII-SV40T (ori-) plasmid into 6 × 107 Molt4 1D10 cells in six cuvettes. After another cycle of cell sorting and recovery, 960 independent clones were analyzed and two positive clones were obtained.
Based on this rat sequence, we found two expressed sequence tag clones of human homolog (accession numbers BG196529 and BG741116). We also found two human genomic clones of chromosome 17 (accession numbers AC023133 and AC110594.2). We connected these sequences into a complete sequence of human PIG-W. We confirmed this sequence by amplification of the coding region from a human cDNA library by polymerase chain reaction and sequencing.
Analysis of GPI Biosynthesis
For labeling with mannose, 106 cells were incubated for 1 h in a medium containing 100 μg/ml glucose and 10 μg/ml tunicamycin (Sigma-Aldrich, St. Louis, MO) and then incubated in the same medium containing 40 μCi/ml d-[2-3H]mannose for 45 min (Hirose et al., 1992). Lipids were extracted and partitioned into n-butanol. To study the effect of YW3548/BE49385A, a terpenoid lactone (Sutterlin et al., 1997), cells were incubated overnight in a medium containing 10 μM BE49385A (a gift from Banyu Pharmaceutical Co., Tokyo, Japan), followed by labeling with mannose. For in vivo labeling with inositol, 106 cells were cultured in inositol-free DMEM supplemented with 10% dialyzed FCS and 20 μCi of myo-[2-3H(N)]inositol for 1 d (Maeda et al., 2001). Glycolipids were separated on Kiesel gel 60 (Merk, Darmstadt, Germany) and detected by a Fuji BAS1500 image analyzer (Fuji Film, Tokyo, Japan).
For analysis of the third step, we prepared membranes from cells preincubated with 5 μg/ml tunicamycin for 2 h by hypotonic lysis and destruction with a Teflon homogenizer. The membranes obtained from Molt4 cells, its derivative cells, and CHOPA10.14 cells (107) were suspended in a buffer (100 mM Tris-HCl, pH 8/1 mM EDTA/protease inhibitors) and incubated for 10 min with 0.1 μg of GlcN-PI (C8), GlcN-PI bearing dioctanoyl-PI (Doerrler et al., 1996) (a gift from Dr. M.A. Lehrman; University of Texas Southwestern Medical Center, Dallas, TX) in 0.03% Triton X-100 and [3H]palmitoyl-CoA.
Enzyme Treatments of Labeled Lipids
Lipids were dissolved in 0.15 ml of a buffer containing either 100 mM Tris-HCl (pH 7.4), 0.1% Triton X-100, and 5 μl (0.1 U) of Bacillus thuringiensis PI-specific phospholipase C (PI-PLC) (Toagosei, Tokyo, Japan); or 100 mM sodium acetate (pH 5.0), 1 mM ZnCl2, 0.1% sodium taurodeoxycholate, and 15 μl (1.7 U) of Jack bean α-mannosidase (Sigma-Aldrich); or 50 mM Tris-HCl (pH 7.4), 10 mM NaCl, 2.5 mM CaCl2, 0.1% Triton X-100, and 15 μl of human serum as a source of GPI-specific phospholipase D (GPI-PLD). After incubation for 16 h at 37°C, the lipids were extracted with n-butanol and analyzed by thin layer chromatography (TLC).
Purification of PIG-W Protein
JY25 cells (107) were transfected with 20 μg of pMEEB-FLAG-rPIG-W-GST, pMEEB-FLAG-rPIG-W-FLAG-HAT, or pMEEB-hPIG-L-GST-FLAG by electroporation at 260 V and 960 μF and cultured for 14 d with 400 μg/ml hygromycin for selection. Proteins were affinity-purified from 108 cells as described previously (Watanabe et al., 2000). The affinity-purified proteins were subjected to 4–20% gradient SDS-PAGE and were either silver stained or used for Western blotting. The proteins were also used for acyltransferase assay.
Acyltransferase Activity Assay With Liposomes Containing Purified PIG-W
A mixture of 3.9 mg of egg yolk phosphatidylcholine and 9.1 mg of phosphatidylethanolamine (Sigma-Aldrich) in 1 ml of chloroform was dried under N2, dissolved in 6.5 ml of 100 mM Tris-HCl (pH 8.0) with 0.5 mM EDTA under sonication in a bath (UT-53N; Sharp, Osaka, Japan) for 5 min. Affinitypurified PIG-W or PIG-L proteins (equivalent to 2.5 × 107 cells) were agitated with 300 μl of a buffer containing liposomes for 3 h at 4°C. A sample of 100 μl of this liposome solution was incubated with 0.1 μg of GlcN-PI (C8) and [3H]palmitoyl-CoA. After 10-min incubation at 37°C, lipids were extracted and analyzed by TLC.
Membrane Topology of PIG-W Protein
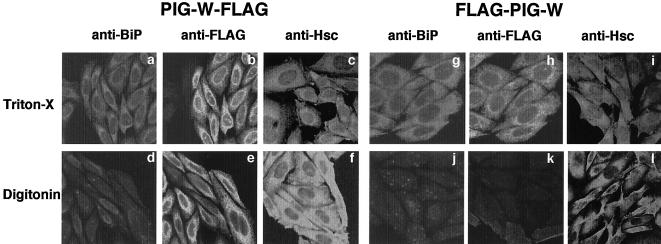
We generated CHO cells stably transfected with pME-neo-FLAG-rPIG-W or pME-neo-rPIG-W-FLAG. Cells were cultured on glass coverslips for 2 d and fixed in 4% paraformaldehyde for 15 min at room temperature. To selectively permeabilize the plasma membrane, cells were incubated in a buffer containing 5 μg/ml digitonin. For complete permeabilization, 0.1% Triton X-100 was added to the blocking solution. Cells were then incubated with 2.5 μg/ml rabbit anti-BiP antibody (ABR–Affinity BioReagents, Golden, CO), mouse anti-FLAG M2 antibody (Sigma-Aldrich), or rat anti-Hsc70 antibody (Stressgen Biotechnology, Victoria, BC, Canada) in 1% bovine serum albumin in phosphate-buffered saline (PBS) for 1 h. After three washes in PBS, cells were incubated with Texas Red-conjugated goat anti-rabbit IgG (Jackson Laboratories, Bar Harbor, ME), Alexa 488-conjugated anti-mouse IgG (Molecular Probes, Eugene, OR) or rhodamine-conjugated goat anti-rat IgG (Chemicon, Temecula, CA) in 1% bovine serum albumin in PBS for 1 h. Slides were mounted in moviol and studied under a confocal microscope (Carl Zeiss, Jena, Germany).
RESULTS
Molt4–1D10 and CHOPA10.14 Cells Are Defective in the Expression of GPI-anchored Proteins Due to the Defective Inositol Acylation
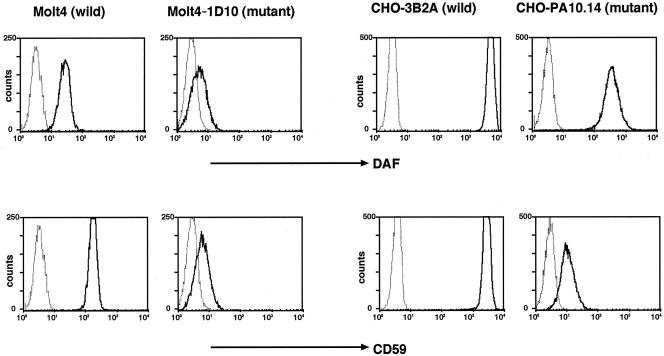
We established Molt4–1D10 mutant from Molt4 cells and CHOPA10.14 mutant from CHO cells. Both mutants were partially defective in their surface expression of GPI-anchored proteins, DAF, and CD59 (Figure 1). PI-PLC removed the partially expressed DAF and CD59 from both cell lines, indicating that they were GPI anchored (our unpublished data).
Figure 1.
Defective surface expression of GPI-anchored proteins on Molt4–1D10 and CHOPA10.14 cells. Wild-type Molt4, mutant Molt4–1D10, wild-type CHO-3B2A, and mutant CHOPA-10.14 cells were stained for DAF (top) and CD59 (bottom) and analyzed in a FACScan. Solid lines, specific monoclonal antibodies; dotted lines, isotype-matched control antibodies.
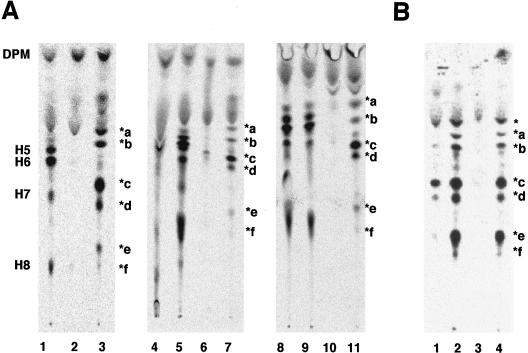
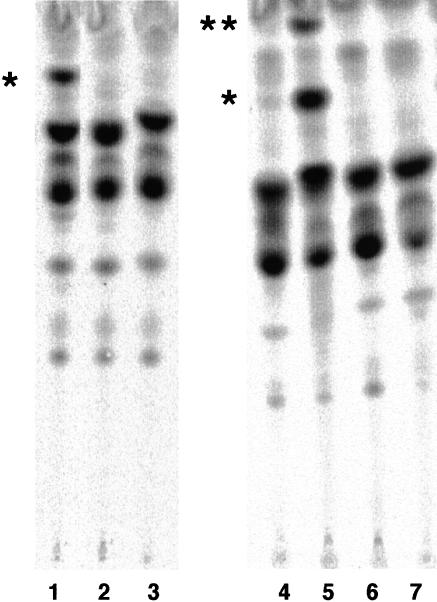
To determine the molecular basis of the defective expression of GPI-anchored proteins on these mutant cells, we analyzed the biosynthesis of the GPI-anchor by metabolic labeling cells with mannose. In contrast to wild-type Molt4 cells (Figure 2A, lane 2), the Molt4–1D10 mutant accumulated abnormal mannolipids, termed *a, *b, *c, *d, *e, and *f (lane 3). Those mannolipids did not align with the mannolipids in class K mutant cells (lane 1), which accumulate various intermediate and mature GPIs, such as H5 to H8, due to a defect in the transfer of GPI to proteins (Yu et al., 1997). CHOPA10.14 cells also accumulated a similar set of abnormal mannolipids (Figure 2B, lanes 2 and 4).
Figure 2.
Accumulation of abnormal, PI-PLC-sensitive GPI species in Molt4–1D10 (A) and CHOPA10.14 (B) cells. (A) Class K mutant K562 (lane 1), wild-type Molt4 (lane 2), and 1D10 mutant (lane 3) cells were cultured with [3H]mannose in the presence of tunicamycin. Mannolipids were analyzed by TLC with a solvent system of chloroform/methanol/H2O (10:10:3). DPM, dolichol-phosphate-mannose; H5, H6, H7, and H8 are according to Hirose et al., 1992. *a–*f, abnormal mannolipids accumulated in 1D10 cells. Labeled lipids of class K cells (lanes 4, 5, 8, and 9) and 1D10 cells (lanes 6, 7, 10, and 11) were treated with GPI-PLD (lanes 4 and 6), buffer for GPI-PLD (lanes 5 and 7), PI-PLC (lanes 8 and 10), or buffer for PI-PLC (lanes 9 and 11). Reextracted lipids were analyzed by TLC. (B) Mannolipids of CHOPA10.14 mutant cells prepared in a similar way to that described in A were treated with GPI-PLD (lane 1), buffer for GPI-PLD (lane 2), PI-PLC (lane 3), or buffer for PI-PLC (lane 4). *, abnormal mannolipid likely to be Man-GlcN-PI (see RESULTS).
All of those abnormal mannolipids were sensitive to GPIPLD (Figure 2A, lanes 6 and 7; B, lanes 1 and 2), like the GPI intermediates accumulated in class K mutant cells (Figure 2A, lanes 4 and 5), confirming that *a to *f were GPI species. In contrast to the GPI intermediates accumulated in class K mutant cells that were resistant to PI-PLC due to the inositol acylation (Figure 2A, lanes 8 and 9), all of *a to *f species were sensitive to PI-PLC (Figure 2A, lanes 10 and 11; B, lanes 3 and 4). These results indicated that the two mutants are defective in their inositol acylation and hence inositol acylation is important for the surface expression of GPI-anchored proteins.
Analysis of Mannolipids Accumulated in Cells Defective in Inositol Acylation
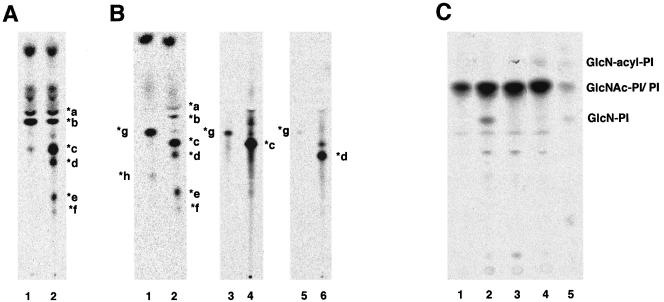
To determine steps for which the acyl chain on inositol is critical, we characterized mannolipids accumulated in the 1D10 mutant. We first tested whether some of the mannolipids have a P-EtN side chain on the first mannose. For this, we used YW3548/BE49386A, which inhibits the addition of P-EtN to the first mannose (Hong et al., 1999). We pretreated mutant cells with this drug for 24 h before metabolic labeling with mannose (Figure 3A). Mannolipids *a and *b remained, whereas *c, *d, *e, and *f disappeared (lanes 1 versus 2), indicating that these four had the P-EtN side chain. Therefore, the inositol acylation is not required for the addition of the P-EtN side chain.
Figure 3.
Characterization of mannolipids accumulated in Molt4–1D10 (A and B) and analysis of early steps in GPI biosynthesis (C). (A) Molt4–1D10 cells cultured in the presence (lane 1) or absence (lane 2) of YW3548/BE49386A for 24 h were metabolically labeled with [3H]mannose. The lipids were analyzed by TLC with a solvent system of chloroform/methanol/H2O (10:10:3). (B) Molt4–1D10 cells were metabolically labeled with [3H]mannose. Lipids were treated with Jack bean α-mannosidase (lane 1) or buffer (lane 2), extracted again, and analyzed by TLC as described in A. Mannolipids *c and *d were eluted from the TLC plate (lanes 4 and 6) and treated with the α-mannosidase and reanalyzed by TLC (lanes 3 and 5). (C) Wild-type Molt4 (lane 1), 1D10 mutant (lane 2), 1D10 transfected with rat PIG-W cDNA (lane 3), and class E mutant (lane 4) cells were cultured in a medium containing myo-[3H]inositol for 1 d. The radiolabeled lipids were analyzed by TLC with a solvent system of chloroform/methanol/1 M NH4OH (10:10:3). Lane 5, standard GlcNAc-PI, GlcN-PI, and GlcN-acylPI generated by incubating membranes of wild-type Molt4 with UDP-[6-3H]GlcNAc. PI and GlcNAc-PI are overlapping.
We then treated the mannolipids with Jack bean α-mannosidase, which removes nonmodified mannoses (Figure 3B). All *a to *f mannolipids were sensitive to the α-mannosidase, and two products, *g and *h, were formed (lane 1 versus 2). Because *a and *b were more hydrophobic than *g and *h, *g and *h were not products of *a and *b. Mannolipids *a and *b must have lost all mannoses upon the α-mannosidase treatment, suggesting that they were GlcNPI–bearing nonmodified mannoses only. Mannolipids *a and *b probably have two and three mannoses, respectively, because CHOPA10.14 cells clearly had one more mannolipid that was more hydrophobic than *a (Figure 2B, lanes 2 and 4, asterisk), most likely GlcN-PI bearing one mannose (Man-GlcN-PI).
To understand relationships between *g, and *c and *d, we isolated *c (Figure 3B, lane 4) and *d (lane 6) from the TLC plate and treated them with the α-mannosidase. Mannolipid *g was derived from both *c (lane 3) and *d (lane 5). This result, taken together with the above-mentioned result that *c and *d have P-EtN on the first mannose (Figure 3A), suggested that *c was Man-(EtNP)Man-GlcN-PI, that *d was Man-Man-(EtNP)Man-GlcN-PI, and that the product *g was (EtNP)Man-GlcN-PI. Mannolipid *e became *h after α-mannosidase treatment, indicating that it had nonmodified mannose, most likely the third mannose. A big difference in the mobilities of *g and *h may be due to the presence of an additional P-EtN in *h. If this is true, *e may have the structures Man-(EtNP)Man-(EtNP)Man-GlcN-PI. Therefore, *b, *d, and *e probably had three mannoses, whereas there was no GPI species that had P-EtN on the third mannose, suggesting that inositol acylation may be critical for the addition of the bridging P-EtN that links GPI to proteins.
Finally, we tested whether a lack of the inositol acylation affects the efficiency of the first mannosylation. We metabolically labeled 1D10 cells and class E mutant cells (defective in synthesis of dolichol-phosphate-mannose; Tomita et al., 1998) with inositol to assess levels of the first three GPI intermediates (Figure 3C). PI was seen mainly in wild-type Molt4 (lane 1), whereas GlcN-PI was accumulated in 1D10 mutant (lane 2). Transfection of the PIG-W cDNA normalized it (lane 3) (see the next section for cloning PIG-W). Class E cells accumulated GlcN-acylPI as expected (Urakaze et al., 1992) (lane 4). These results indicate that inositol acylation is not essential for but accelerates the first mannosylation.
Cloning Rat PIG-W cDNA
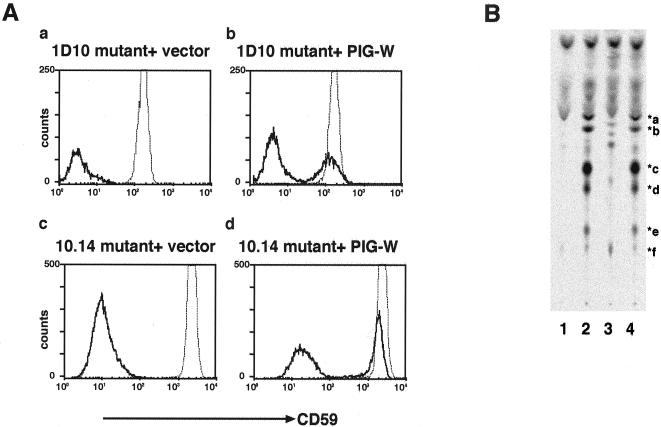
We isolated a cDNA of the rat PIG-W gene that restored the surface expression of GPI-anchored proteins on Molt4–1D10 cells by means of expression cloning (Figure 4A, a and b). The same cDNA restored the surface expression of CD59 on CHOPA10.14 cells (c and d). Rat PIG-W cDNA also normalized GPI biosynthesis in the 1D10 mutant as revealed by metabolic labeling cells with mannose (Figure 4B). Transfection of a PIG-W cDNA (lane 3) but not an empty vector (lane 4) resulted in the disappearance of the accumulated mannolipids seen in the 1D10 mutant (lane 2).
Figure 4.
Molt4–1D10 and CHOPA10.14 cells are defective in PIG-W. (A) Rat PIG-W cDNA restored the surface expression of CD59 on Molt4–1D10 and CHOPA10.14 cells. 1D10 mutant transfected with an empty vector (a) or PIG-W (b) and CHOPA10.14 mutant transfected with an empty vector (c) or PIG-W (d) were stained 2 d after transfection. Solid lines, transfectants; dotted lines, wild-type cells. (B) Normalized GPI biosynthesis in 1D10 cells transfected with PIG-W cDNA. Wild-type Molt4 (lane 1), 1D10 mutant (lane 2), 1D10 transfected with rat PIG-W cDNA (lane 3), and 1D10 mutant transfected with an empty vector (lane 4) were labeled with [3H]mannose and lipids analyzed by TLC.
We then analyzed PIG-W mRNA in Molt4–1D10 cells by reverse transcription-polymerase chain reaction to see whether PIG-W is mutated. A cDNA of the same size was amplified from samples of wild-type and 1D10 mutant cells (our unpublished data). Direct sequencing of the band revealed a point mutation at nucleotide 281 that changed leucine to a stop codon, indicating that PIG-W is the defective gene in the 1D10 mutant.
Characteristics of PIG-W Protein
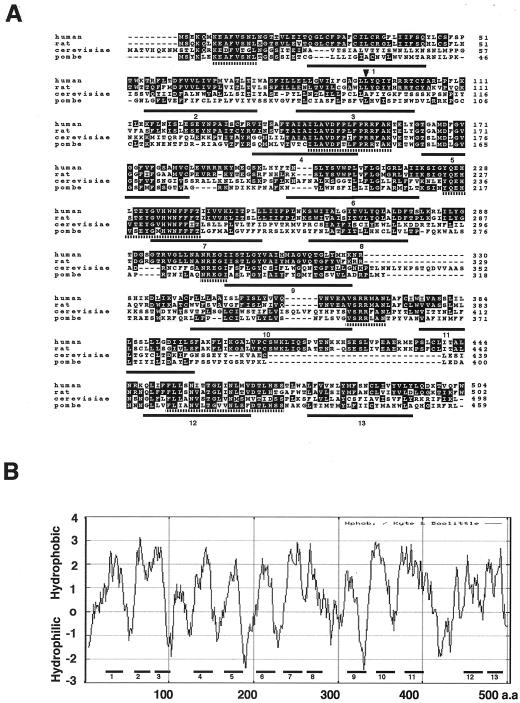
An open reading frame of rat PIG-W cDNA consisted of 1506 base pairs encoding 502 amino acids (Figure 5A). Searching databases, we found a human PIG-W gene (an intron-less gene mapped to chromosome 17q) that encoded 504 amino acids with 77% identity to rat PIG-W (the DDBJ/EMBL/GenBank accession numbers of rat and human PIG-W cDNAs are AB097817 and AB097818, respectively).
Figure 5.
(A) Alignment of amino acid sequences of PIG-W homologs of human, rat, S. cerevisiae, and S. pombe. Black and gray boxes indicate identical and conserved amino acids, respectively. Six regions highly conserved in four species are indicated by broken underlines. Positions of 13 predicted transmembrane domains of S. pombe PIG-W are indicated by numbered underlines. An arrowhead indicates leucine changed to a stop codon in 1D10 mutant. (B) Hydropathic profile of human PIG-W. Hydrophobicity was calculated with a window length of 17 (Kyte and Doolittle, 1982). Positions of 13 predicted transmembrane domains are indicated by numbered thick bars.
We also found yeast PIG-W homologs. Saccharomyces cerevisiae PIG-W (YJL091C) consists of 498 amino acids and has 28% amino acid identity to human PIG-W. Schizosaccharomyces pombe PIG-W (accession no. CAB59690) consists of 459 amino acids with 28% identity to human PIG-W (Figure 5A). Human PIG-W and its homologs had no significant sequence homology to other proteins, although they did share six well conserved regions (broken underlines in Figure 5A). The S. cerevisiae and S. pombe PIG-W homologs restored the surface expression of CD59 on Molt4–1D10 and CHOPA10.14 cells after transfection, indicating that they are functional PIG-W homologs (our unpublished data).
The hydropathic profile of human PIG-W shows multiple hydrophobic regions (Figure 5B). Analysis with the TMpred program (Smith et al., 1996a) suggested the presence of 13 transmembrane domains in both human (Figure 5B, numbered bars) and S. pombe PIG-W (Figure 5A, numbered underlines). Based on comparison of locations of predicted transmembrane domains and the regions conserved among PIG-W homologs, presumably functionally important regions, we speculated that all the conserved regions are on the same side of the membrane as the N terminus (see below for the membrane orientation of the N terminus).
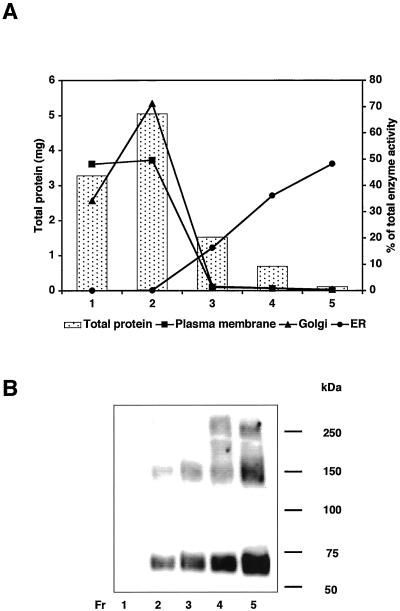
We then analyzed subcellular localization of PIG-W protein. We transfected a cDNA of glutathione S-transferase (GST)–tagged PIG-W into JY25 cells and fractionated nuclei-free lysates into the endoplasmic reticulum (ER), the Golgi apparatus, and plasma membranes by sucrose density gradient centrifugation (Figure 6A). Western blotting with anti-GST antibody revealed PIG-W mainly in fractions 4 and 5, corresponding to the ER (Figure 6B) (see the next section for an explanation for the higher molecular weight bands). Fractions 1 and 2 containing the plasma membranes and the Golgi apparatus had none and only a small amount of PIG-W, respectively, indicating that PIG-W is an ER protein. There is no known ER localization signal sequence in PIG-W (Figure 5A).
Figure 6.
ER localization of PIG-W. JY25 cells expressing GST-tagged PIG-W were hypotonically lysed and the postnuclear supernatant fractionated by sucrose density gradient centrifugation. Fractions 1–5 were characterized by protein content (bars) and organelle marker enzymes (alkaline phosphodiesterase I for the plasma membrane, α-mannosidase II for the Golgi apparatus, and dolicholphosphate-mannose synthase for the ER) (A) and by Western blotting with anti-GST antibody (B). The enzyme activities in fractions are shown as percentages of the total.
Because PIG-W resides in the ER, the site of GPI biosynthesis, and because both S. cerevisiae and S. pombe PIG-W homologs were functional in human and hamster mutant cells, it is very likely that PIG-W is directly involved in inositol acylation.
PIG-W Is Most Likely the Acyltransferase
To set up an assay for inositol acyltransferase, we used a synthetic substrate GlcN-PI(C8). It was reported that GlcNPI(C8) acted as a substrate of acyltransferase present in the membrane of mammalian cells, receiving the palmitoyl chain from [3H]palmitoyl-CoA (Doerrler et al., 1996). Microsomal membranes of wild-type CHO (Figure 7, lane 1), but not the CHOPA10.14 mutant (lane 2), generated a specific spot (asterisk) in the presence but not absence (lane 3) of GlcN-PI(C8). Wild-type Molt4 (lane 4) inefficiently generated a similar spot (lane 4), whereas Molt4–1D10 transfected with PIG-W efficiently generated it in the presence (lane 5) but not absence (lane 6) of GlcN-PI(C8). An extra spot formed when acyltransferase activity was very high (double asterisks in lane 5). Nontransfected 1D10 did not make the specific spot (lane 7), indicating that this specific spot was GlcN-acylPI(C8). Therefore, a combination of GlcN-PI(C8) and [3H]palmitoyl-CoA should be useful to assay inositol acyltransferase.
Figure 7.
CHOPA10.14 and Molt4–1D10 are defective in inositol acylation of a synthetic substrate GlcN-PI(C8). The membranes of wild-type CHO (lanes 1 and 3) and CHOPA10.14 (lanes 2) cells were incubated with [3H]palmitoyl-CoA in the presence (lanes 1 and 2) or absence (lane 3) of GlcN-PI(C8). The membranes of wild-type Molt4 (lane 4), Molt4–1D10 transfected with PIG-W (lanes 5 and 6), and 1D10 mutant (lane 7) were incubated with [3H]palmitoyl-CoA in the presence (lanes 4, 5, and 7) or absence (lane 6) of GlcN-PI(C8). After 10-min-incubation, lipids were analyzed by TLC with a solvent system of chloroform/methanol/0.25% KCl (55:45:10). *, GlcN-acylPI(C8); **, GlcN-PI(C8)-dependent extraspot of unknown structure.
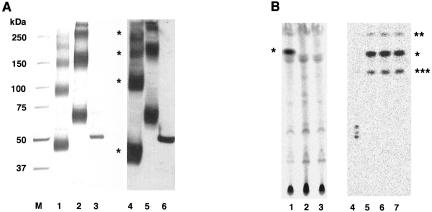
To determine whether PIG-W is acyltransferase, we affinity purified PIG-W from human JY25 cells expressing rat PIG-W tagged with FLAG and HAT, or with FLAG and GST. As a control, PIG-L bearing FLAG and GST tags was similarly affinity purified. Both PIG-W preparations showed four silver-stained bands after SDS-PAGE under reducing conditions (Figure 8A, lanes 1 and 2). Bands of FLAG- and HAT-tagged PIG-W corresponded to 45, 90, 140–150, and 200 kDa (lane 1). All of them reacted with anti-FLAG antibodies on Western blotting (lane 4). The FLAG- and GST-tagged PIG-W showed four bands at 70, 150, 250, and >300 kDa (lane 2), all of which reacted with anti-FLAG antibodies (lane 5). The size difference between the two PIG-W preparations corresponded to the size difference between HAT (2 kDa) and GST (25 kDa). It is very likely that the four bands represented mono-, di-, tri-, and tetrameric PIG-W, respectively. The affinity-purified PIG-L showed a single band of an expected size (lanes 3 and 6).
Figure 8.
The PIG-W gene encodes acyltransferase. (A) FLAG-PIG-W-FLAG-HAT (lanes 1 and 4), FLAG-PIG-W-GST (lanes 2 and 5), and PIG-LGST-FLAG (lanes 3 and 6) were isolated by two-step affinity purification from the lysates of transfected JY25 cells. M, molecular size markers; lanes 1–3, silver staining; lanes 4–6, Western blotting with anti-FLAG antibody. (B) The purified FLAG-PIG-W-GST (lanes 1 and 3) and PIG-L-GST-FLAG (lane 2) proteins were incubated with [3H]palmitoyl-CoA in the presence (lanes 1 and 2) or absence (lane 3) of GlcN-PI(C8). After 10-min-incubation, lipids were analyzed by TLC with a solvent system of chloroform/methanol/0.25% KCl (55:45:10). GlcN-acylPI(C8) generated (asterisk) was treated overnight with GPI-PLD (lane 4), buffer for GPI-PLD (lane 5), PI-PLC (lane 6), or buffer for PI-PLC (lane 7), extracted again, and analyzed by TLC.
We then measured the acyltransferase activity of the affinity-purified PIG-W by using GlcN-PI(C8) and [3H]palmitoyl-CoA. The specific product (asterisk) occurred after incubation with the FLAG- and GST-tagged PIG-W in the presence of GlcN-PI(C8) (Figure 8B, lane 1). This spot did not occur after incubation with PIG-L (lane 2) nor with the PIG-W in the absence of GlcN-PI(C8) (lane 3). To confirm that the product was in fact GlcN-acylPI(C8), the spot was extracted from the TLC plate and treated with GPI-PLD and PI-PLC. After overnight incubation with buffer alone, the sample gave two extraradioactive spots (double and triple asterisks) (lanes 5 and 7), suggesting migration of the palmitoyl group during incubation (Costello and Orlean, 1992; Doerrler et al., 1996). The one with double asterisks had mobility similar to that of the second spot seen with membrane-bound inositol acyltransferase (Figure 7, lane 5, double asterisks). The product was sensitive to GPI-PLD having generated a set of much less hydrophobic species (Figure 8B, lane 4). This is consistent with the prediction that GlcN-acylPI(C8) would yield radioactive GlcN-acyl-inositol. The reason for the generation of a cluster of three spots is unclear but may be related to migration of the acyl chain. The product was resistant to PI-PLC as expected (lane 6). Therefore, affinity-purified PIG-W had acyltransferase activity, suggesting that PIG-W is most likely an acyltransferase itself.
Luminal Orientation of the N and Cytoplasmic Orientation of the C Termini of PIG-W
We then determined the membrane orientation of the N and C termini of PIG-W. We generated rat PIG-W tagged with FLAG epitope at either the N or the C termini, confirmed that these tagged PIG-Ws are functional as determined by complementation of CHOPA10.14 cells, and then transfected them into CHO cells (Figure 9). After permeabilization of both the plasma and ER membranes by Triton X-100 or after the selective permeabilization of only the plasma membrane by digitonin, we stained cells for a FLAG tag and an authentic ER luminal protein, BiP. We also stained an authentic cytosolic protein Hsc70. The FLAG at the C termini of PIG-W was accessed by an antibody after permeabilization of just the plasma membrane (Figure 9e). Cells with the FLAG at the N terminus were stained only after permeabilization of the ER membrane (h versus k), similarly to BiP (g versus j). Therefore, the N terminus was luminally and C termini was cytoplasmically oriented.
Figure 9.
Membrane orientation of the N and C termini of PIG-W. Rat PIG-W tagged at the C termini (PIG-W-FLAG) (a–f) or the N terminus (FLAG-PIG-W) (g–l) were expressed in CHO cells. After permeabilization of both the plasma membrane and the ER membrane (a–c and g–i), or selective permeabilization of the plasma membrane (d–f and j–k), cells were stained for tagged PIG-W by anti-FLAG antibody (b, e, h, and k), for an endogenous ER luminal protein BiP (a, d, g, and j) and a cytosolic protein Hsc70 (c, f, I, and l).
Combining this result with the prediction that the regions conserved among species and the N terminus have the same membrane orientation (Figure 5B), we speculated that the conserved regions may face the luminal side; hence, the acyltransfer probably occurs on the luminal side.
DISCUSSION
PIG-W Accounts for Most, If Not All, of the Inositol Acylation Activity
We isolated GPI-anchor–deficient cell lines, Molt4–1D10 and CHOPA10.14, and demonstrated that they are defective in the transfer of the acyl chain to inositol. We obtained rat PIG-W cDNA that restored GPI biosynthesis in Molt4–1D10 and CHOPA10.14 cells. Molt4–1D10 cells had a nonsense mutation at nucleotide 281 in the PIG-W gene. The epitopetagged PIG-W, affinity purified from transfected human cells, had acyl-CoA–dependent inositol acyltransferase activity in vitro, indicating that Molt4–1D10 is defective in inositol acyltransferase.
Molt4–1D10 and CHOPA10.14 cells had no detectable inositol acylated GPI precursors. Therefore, PIG-W accounts for most, if not all, of the inositol acylation activity. Molt4–1D10 expressed only 20 and a few percent of normal levels of DAF and CD59, respectively, and CHOPA10.14 cells expressed only a few and <1% of DAF and CD59, respectively. These residual proteins were GPI anchored. It is unclear whether there is a second minor mechanism for the inositol acylation that may be responsible for the residual GPI anchoring (Stevens and Zhang, 1994) or whether noninositolacylated GPI can, albeit inefficiently, be transferred to the proteins.
Inositol Acylation Is Not Essential for Flipping of GPI but May Be Critical for the Addition of the Bridging PEtN to the Third Mannose
Both Molt4–1D10 and CHOPA10.14 cells accumulated a number of mannosylated noninositol-acylated GPI species. Considering that GPI should flip from the cytoplasmic side to the luminal side before the first mannosylation, this result indicated that inositol acylation is not essential for flipping. This is consistent with a report that the additions of three mannoses occurred in T. brucei even when inositol acylation was inhibited by phenylmethylsulfonyl fluoride (Guther et al., 1994).
That the first mannosylation occurs without inositol acylation was a surprising finding, and contrary to our current concept that inositol acylation is a prerequisite for the addition of the first mannose in mammalian cells (Urakaze et al., 1992; Smith et al., 1997). It should still hold that inositol acylation precedes the first mannosylation in mammalian cells (Doerrler et al., 1996). The first mannosylation by PIG-M (Maeda et al., 2001) seemed to be more efficient with inositol acylation because GlcN-PI was accumulated in Molt4–1D10 cells and this accumulation disappeared after transfection of PIG-W cDNA.
Mannolipids *b, *d, and *e seem to have three mannoses, suggesting that inositol acylation is not essential for the second mannosyltransferase and the PIG-B–mediated third mannosylation (Takahashi et al., 1996). Mannolipids *c, *d, *e, and *f had the P-EtN side chain on the first mannose. Therefore, inositol acylation is not essential for the action of PIG-N, the enzyme that attaches this side chain (Hong et al., 1999). It may be that inositol-acylated species are better substrates of these three enzymes because nonacylated species were accumulated.
On the contrary, we did not find GPI having P-EtN on the third mannose in these mutants. Therefore, inositol acylation may be critical for the addition of the bridging P-EtN to the third mannose, suggesting that the substrate specificity of the P-EtN transferase consisting of PIG-O and PIG-F (Hong et al., 2000) is restricted to the inositol acylated intermediate.
Comparison of GPI Biosynthetic Pathways in Mammalian Cells and T. brucei
The first mannosyltransferase (MT1) of T. brucei mannosylates GlcN-PI efficiently, whereas mammalian MT1 prefers GlcN-acylPI (Doerrler et al., 1996; Smith et al., 1996b). This different substrate specificity of MT1 has been a rational basis for the development of trypanosome-specific inhibitors. In fact, a substrate analog GlcN-(2-O-hexadecyl)PI, in which position 2 of inositol is modified by C16 alkyl instead of acyl chain, acts as an inhibitor of T. brucei MT1 but not mammalian MT1 (Smith et al., 1997). The present study demonstrated that mammalian MT1 clearly uses GlcN-PI as a substrate and is not strictly dependent upon inositol acylation. Therefore, substrates of mammalian and T. brucei MT1 at least partially overlap, rather than clearly differ from each other. It is unknown whether T. brucei MT1 can mannosylate GlcN-acylPI partly because chemical synthesis of GlcN-acylPI is not practical due to acyl migration. PIG-M, an ER membrane protein with multiple transmembrane domains, is mammalian MT1 (Maeda et al., 2001). T. brucei has a PIG-M homolog with 30% amino acid identity to human PIG-M (Maeda et al., 2001).
Regarding substrate specificity of inositol acyltransferases, it was reported that the T. brucei enzyme acts on Man-GlcN-PI but not GlcN-PI (Guther and Ferguson, 1995), whereas mammalian inositol-acyltransferase acts on GlcN-PI (Urakaze et al., 1992; Stevens and Zhang, 1994; Doerrler et al., 1996). These specificities are complementary to the substrate specificity of MT1, accounting for the different orders of two reactions. It is unclear, however, whether T. brucei has any ability to generate GlcN-acylPI in vivo. Disruption or RNAi (Wang and Englund, 2001) of PIG-M in procyclic T. brucei need to be undertaken to determine whether indeed the first mannosylation is a prerequisite for inositol acylation. In bloodstream form T. brucei, GPI intermediates bearing one, two, and three mannoses are in equilibrium between the inositol-acylated and the-nonacylated forms (Guther and Ferguson, 1995). This is due to the actions of inositol acyltransferase and inositol deacylase (Guther and Ferguson, 1995). All three mannosylated GlcN-PI may be substrates of T. brucei PIG-W, or alternatively there are more than one inositol acyltransferases with different substrate specificities. With respect to the inositol deacylation, one enzyme has recently been characterized and the presence of yet another inositol deacylase has been proposed (Guther et al., 2001).
It was reported that inositol acylation in T. brucei is critical for the transfer of the P-EtN that bridges the GPI-anchor to the protein, i.e., the acyl chain on inositol is a requirement for ethanolaminephosphotransferase (Guther and Ferguson, 1995). The present study suggested that this may be also true in a mammalian system, where the transfer of the bridging P-EtN is mediated by a complex of PIG-O and PIG-F, in which PIG-F stabilizes catalytic PIG-O (Hong et al., 2000). It is suggested that T. brucei may have a similar enzyme.
Membrane Orientation of Inositol Acylation
Comparison of amino acid sequences of mammalian PIG-W and PIG-W homologs of various organisms revealed six well conserved regions. Sites important for inositol acylation are very likely included in these regions. Based on the positions of predicted transmembrane domains and the luminal orientation of the N terminus of PIG-W, we predicted that all the well conserved regions face the luminal side of the ER. If this prediction is correct, inositol acylation may occur on the luminal side. Whereas the presence of acyl-CoA on the luminal side has not been demonstrated, there may be a mechanism for translocation of acyl-CoA across the ER membrane. An analysis of functional sites of PIG-W and determination of their membrane orientation are required to prove the luminal orientation of inositol acylation.
Acknowledgments
We thank Dr. Kazuhito Ohishi for discussion, Kohjiro Nakamura for help in cell sorting, and Keiko Kinoshita and Fumiko Ishii for technical assistance. This work was supported by grants from the Ministry of Education, Science, Sports, Culture and Technology of Japan.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–03–0193. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-03-0193.
† These authors contributed equally to this work.
Abbreviations used: DAF, decay accelerating factor; GlcN, glucosamine; GlcNAc, N-acetylglucosamine; GlcN-PI(C8), glucosamine-PI bearing dioctanoyl-PI; GPI, glycosylphosphatidylinositol; GPI-PLD, glycosylphosphatidylinositol-specific phospholipase D; MT1, first mannosyltransferase; P-EtN, phosphoethanolamine; PI, phosphatidylinositol; PI-PLC, phosphatidylinositol-specific phospholipase C.
Note added to proof. Two articles describing PIG-W homologue of Saccharomyces cerevisiae have appeared recently: Tsukahara, K., Hata, K., Nakamoto, K., Sagane, K., Watanabe, N., Kuromitsu, J., Kai, J., Tsuchiya, M., Ohba, F., Jigami, Y., Yoshimatsu, K., and Nagasu, T. (2003) Medical genetics approach towards identifying the molecular target of a navel inhibitor of fungal cell wall assembly. Mol. Microbiol. 48, 1029–1042; Umemura, M., Okamoto, M., Nakayama, K., Sagane, K., Tsukahara, K., Hata, K., and Jigami, Y. (2003) GWT1 gene is required for inositol acylation of glycosylphosphatidylinositol anchors in yeast. J. Biol. Chem. 278, 23639–23647.
References
- Buckley, J.T. (1999). The channel-forming toxin aerolysin. In: The Comprehensive Sourcebook of Bacterial Protein Toxins, ed. J.E. Alouf and J.H. Freer, London: Academic Press, 362–372.
- Chen, R., Walter, E.I., Parker, G., Lapurga, J.P., Millan, J.L., Ikehara, Y., Udenfriend, S., and Medof, M.E. (1998). Mammalian glycophosphatidylinositol anchor transfer to proteins and posttransfer deacylation. Proc. Natl. Acad. Sci. USA 95, 9512–9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello, L.C., and Orlean, P. (1992). Inositol acylation of a potential glycosyl phosphoinositol anchor precursor from yeast requires acyl coenzyme A. J. Biol. Chem. 267, 8599–8603. [PubMed] [Google Scholar]
- Doerrler, W.T., Ye, J., Falck, J.R., and Lehrman, M.A. (1996). Acylation of glucosaminyl phosphatidylinositol revisited. J. Biol. Chem. 271, 27031–27038. [DOI] [PubMed] [Google Scholar]
- Ferguson, M.A. (1999). The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J. Cell Sci. 112, 2799–2809. [DOI] [PubMed] [Google Scholar]
- Ferguson, M.A.J. (2000). Glycosylphosphatidylinositol biosynthesis validated as a drug target for African sleeping sickness. Proc. Natl. Acad. Sci. USA 97, 10673–10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda, D.C., and Davidson, E.A. (1999). Protein glycosylation in the malaria parasite. Parasitol. Today 15, 147–152. [DOI] [PubMed] [Google Scholar]
- Guther, M.L., and Ferguson, M.A. (1995). The role of inositol acylation and inositol deacylation in GPI biosynthesis in Trypanosoma brucei. EMBO J. 14, 3080–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guther, M.L., Leal, S., Morrice, N.A., Cross, G.A., and Ferguson, M.A. (2001). Purification, cloning and characterization of a GPI inositol deacylase from Trypanosoma brucei. EMBO J. 20, 4923–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guther, M.L., Masterson, W.J., and Ferguson, M.A. (1994). The effects of phenylmethylsulfonyl fluoride on inositol-acylation and fatty acid remodeling in African trypanosomes. J. Biol. Chem. 269, 18694–18701. [PubMed] [Google Scholar]
- Hirose, S., Prince, G.M., Sevlever, D., Ravi, L., Rosenberry, T.L., Ueda, E., and Medof, M.E. (1992). Characterization of putative glycoinositol phospholipid anchor precursors in mammalian cells. Localization of phosphoethanolamine. J. Biol. Chem. 267, 16968–16974. [PubMed] [Google Scholar]
- Hollander, N., Selvaraj, P., and Springer, T.A. (1988). Biosynthesis and function of LFA-3 in human mutant cells deficient in phosphatidylinositol-anchored proteins. J. Immunol. 141, 4283–4290. [PubMed] [Google Scholar]
- Hong, Y., Maeda, Y., Watanabe, R., Inoue, N., Ohishi, K., and Kinoshita, T. (2000). Requirement of PIG-F and PIG-O for transferring phosphoethanolamine to the third mannose in glycosylphosphatidylinositol. J. Biol. Chem. 275, 20911–20919. [DOI] [PubMed] [Google Scholar]
- Hong, Y., Maeda, Y., Watanabe, R., Ohishi, K., Mishkind, M., Riezman, H., and Kinoshita, T. (1999). Pig-n, a mammalian homologue of yeast Mcd4p, is involved in transferring phosphoethanolamine to the first mannose of the glycosylphosphatidylinositol. J. Biol. Chem. 274, 35099–35106. [DOI] [PubMed] [Google Scholar]
- Hong, Y., Ohishi, K., Inoue, N., Kang, J.Y., Shime, H., Horiguchi, Y., van der Goot, F.G., Sugimoto, N., and Kinoshita, T. (2002). Requirement of N-glycan on GPI-anchored proteins for efficient binding of aerolysin but not Clostridium septicum α-toxin. EMBO J. 21, 5047–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman, R. (1988). Somatic genetic analysis of the expression of cell surface molecules. Trends Genet. 4, 5–8. [DOI] [PubMed] [Google Scholar]
- Ikezawa, H. (2002). Glycosylphosphatidylinositol (GPI)-anchored proteins. Biol. Pharm. Bull. 25, 409–417. [DOI] [PubMed] [Google Scholar]
- Kinoshita, T., and Inoue, N. (2000). Dissecting and manipulating the pathway for glycosylphosphatidylinositol-anchor biosynthesis. Curr. Opin. Chem. Biol. 4, 632–638. [DOI] [PubMed] [Google Scholar]
- Kyte, J., and Doolittle, R.F. (1982). A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157, 105–132. [DOI] [PubMed] [Google Scholar]
- Maeda, Y., Tomita, S., Watanabe, R., Ohishi, K., and Kinoshita, T. (1998). DPM2 regulates biosynthesis of dolichol phosphate-mannose in mammalian cells: correct subcellular localization and stabilization of DPM1, and binding of dolichol phosphate. EMBO J. 17, 4920–4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda, Y., Watanabe, R., Harris, C.L., Hong, Y., Ohishi, K., Kinoshita, K., and Kinoshita, T. (2001). PIG-M transfers the first mannose to glycosylphosphatidylinositol on the lumenal side of the ER. EMBO J. 20, 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville, M.J., and Menon, A.K. (2000). Recent developments in the cell biology and biochemistry of glycosylphosphatidylinositol lipids. Mol. Membr. Biol. 17, 1–16. [DOI] [PubMed] [Google Scholar]
- Nagamune, K., et al. (2000). Critical roles of glycosylphosphatidylinositol for Trypanosoma brucei. Proc. Natl. Acad. Sci. USA 97, 10336–10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, N., Inoue, N., Watanabe, R., Takahashi, M., Takeda, J., Stevens, V.L., and Kinoshita, T. (1997). Expression cloning of PIG-L, a candidate N-acetylglucosaminyl-phosphatidylinositol deacetylase. J. Biol. Chem. 272, 15834–15840. [DOI] [PubMed] [Google Scholar]
- Ohishi, K., Inoue, N., Maeda, Y., Takeda, J., Riezman, H., and Kinoshita, T. (2000). Gaa1p and gpi8p are components of a glycosylphosphatidylinositol (GPI) transamidase that mediates attachment of GPI to proteins. Mol. Biol. Cell 11, 1523–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, K., and Toomre, D. (2000). Lipid rafts and signal transduction. Nat. Rev. Mol. Cell. Biol. 1, 31–39. [DOI] [PubMed] [Google Scholar]
- Smith, R.F., Wiese, B.A., Wojzynski, M.K., Davison, D.B., and Worley, K.C. (1996a). BCM Search Launcher-an integrated interface to molecular biology data base search and analysis services available on the World Wide Web. Genome Res. 6, 454–462. [DOI] [PubMed] [Google Scholar]
- Smith, T.K., Cottaz, S., Brimacombe, J.S., and Ferguson, M.A. (1996b). Substrate specificity of the dolichol phosphate mannose:glucosaminyl phosphatidylinositol α1–4-mannosyltransferase of the glycosylphosphatidylinositol biosynthetic pathway of African trypanosomes. J. Biol. Chem. 271, 6476–6482. [DOI] [PubMed] [Google Scholar]
- Smith, T.K., Sharma, D.K., Crossman, A., Dix, A., Brimacombe, J.S., and Ferguson, M.A. (1997). Parasite and mammalian GPI biosynthetic pathways can be distinguished using synthetic substrate analogues. EMBO J. 16, 6667–6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, V.L., and Zhang, H. (1994). Coenzyme A dependence of glycosylphosphatidylinositol biosynthesis in a mammalian cell-free system. J. Biol. Chem. 269, 31397–31403. [PubMed] [Google Scholar]
- Sutterlin, C., Horvath, A., Gerold, P., Schwarz, R.T., Wang, Y., Dreyfuss, M., and Riezman, H. (1997). Identification of a species-specific inhibitor of glycosylphosphatidylinositol synthesis. EMBO J. 16, 6374–6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, M., Inoue, N., Ohishi, K., Maeda, Y., Nakamura, N., Endo, Y., Fujita, T., Takeda, J., and Kinoshita, T. (1996). PIG-B, a membrane protein of the endoplasmic reticulum with a large lumenal domain, is involved in transferring the third mannose of the GPI anchor. EMBO J. 15, 4254–4261. [PMC free article] [PubMed] [Google Scholar]
- Tiede, A., Bastisch, I., Schubert, J., Orlean, P., and Schmidt, R.E. (1999). Biosynthesis of glycosylphosphatidylinositols in mammalian and unicellular microbes. Biol. Chem. 380, 503–523. [DOI] [PubMed] [Google Scholar]
- Tomita, S., Inoue, N., Maeda, Y., Ohishi, K., Takeda, J., and Kinoshita, T. (1998). A homologue of Saccharomyces cerevisiae Dpm1p is not sufficient for synthesis of dolichol-phosphate-mannose in mammalian cells. J. Biol. Chem. 273, 9249–9254. [DOI] [PubMed] [Google Scholar]
- Urakaze, M., Kamitani, T., DeGasperi, R., Sugiyama, E., Chag, H.M., Warren, C.D., and Yeh, E.T.H. (1992). Identification of a missing link in glycosylphosphatidylinositol anchor biosynthesis in mammalian cells. J. Biol. Chem. 267, 6459–6462. [PubMed] [Google Scholar]
- Vidugiriene, J., and Menon, A.K. (1993). Early lipid intermediates in glycosylphosphatidylinositol anchor assembly are synthesized in the ER and located in the cytoplasmic leaflet of the ER membrane bilayer. J. Cell Biol. 121, 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., and Englund, P.T. (2001). RNA interference of a trypanosome topoisomerase II causes progressive loss of mitochondrial DNA. EMBO J. 20, 4674–4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, R., Kinoshita, T., Masaki, R., Yamamoto, A., Takeda, J., and Inoue, N. (1996). PIG-A and PIG-H, which participate in glycosylphosphatidylinositol anchor biosynthesis, form a protein complex in the endoplasmic reticulum. J. Biol. Chem. 271, 26868–26875. [DOI] [PubMed] [Google Scholar]
- Watanabe, R., Murakami, Y., Marmor, M.D., Inoue, N., Maeda, Y., Hino, J., Kangawa, K., Julius, M., and Kinoshita, T. (2000). Initial enzyme for glycosylphosphatidylinositol biosynthesis requires PIG-P and is regulated by DPM2. EMBO J. 19, 4402–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J., Nagarajan, S., Knez, J.J., Udenfriend, S., Chen, R., and Medof, M.E. (1997). The affected gene underlying the class K glycosylphosphatidylinositol (GPI) surface protein defect codes for the GPI transamidase. Proc. Natl. Acad. Sci. USA 94, 12580–12585. [DOI] [PMC free article] [PubMed] [Google Scholar]