Abstract
Endoplasmic reticulum (ER) stress in the budding yeast Saccharomyces cerevisiae triggers Ca2+ influx through a plasma membrane channel composed of Cch1 and Mid1. This response activates calcineurin, which helps to prevent cell death during multiple forms of ER stress, including the response to azole-class antifungal drugs. Herein, we show that ER stress activates the cell integrity mitogen-activate protein kinase cascade in yeast and that the activation of Pkc1 and Mpk1 is necessary for stimulation of the Cch1-Mid1 Ca2+ channel independent of many known targets of Mpk1 (Rlm1, Swi4, Swi6, Mih1, Hsl1, and Swe1). ER stress generated in response to miconazole, tunicamycin, or other inhibitors also triggered a transient G2/M arrest that depended upon the Swe1 protein kinase. Calcineurin played little role in the Swe1-dependent cell cycle arrest and Swe1 had little effect on calcineurin-dependent avoidance of cell death. These findings help to clarify the interactions between Mpk1, calcineurin, and Swe1 and suggest that the calcium cell survival pathway promotes drug resistance independent of both the unfolded protein response and the G2/M cell cycle checkpoint.
INTRODUCTION
The endoplasmic reticulum (ER) functions in the biogenesis of lipids and disulfide-bonded glycoproteins that are retained or trafficked to other parts of the cell. Cells respond to insufficient glycosylation, disulfide bonding, processing, folding, or assembly of proteins in the ER through the well-characterized unfolded protein response (UPR), which involves the Ire1 receptor/kinase/endoribonuclease in yeast (Sidrauski et al., 1998). We recently demonstrated the existence of an Ire1-independent response to these forms of ER stress that results in stimulation of the Cch1-Mid1 Ca2+ channel in the plasma membrane of yeast cells, elevation of cytosolic free Ca2+, and activation of the Ca2+/calmodulin-dependent protein phosphatase calcineurin (Bonilla et al., 2002). Mutants lacking Cch1, Mid1, calmodulin, or calcineurin function execute early steps of the UPR in response to ER stress but rapidly lose viability, in striking contrast to wild-type cells and ire1 mutants, which tolerate ER stress for long periods. This calcium cell survival (CCS) pathway therefore plays a pivotal role in the response to ER stress independent of the UPR. Unlike the UPR, the CCS pathway responds to azole-class inhibitors of ergosterol biosynthesis in the ER (Bonilla et al., 2002). Many pathogenic fungi in humans also use the CCS pathway for resistance to azole-class drugs, the most commonly prescribed treatment for fungal infections (Del Poeta et al., 2000; Marchetti et al., 2000a,b; Bonilla et al., 2002; Cruz et al., 2002). These findings raise the possibility that specific inhibitors of fungal Ca2+ channels, calmodulin, calcineurin, or other as yet unknown components of the CCS pathway could greatly improve the efficacy of existing antifungal therapies. Nonimmunosupressive derivatives of FK506 and cyclosporin A that are unable to inhibit human calcineurin (Liu et al., 1991) but still able to inhibit fungal calcineurin might be useful in this regard (Cruz et al., 2000).
Calcineurin independently regulates several factors in yeast. The activity of Vcx1, a vacuolar H+/Ca2+ exchanger, seems to be strongly inhibited by calcineurin signaling (Cunningham and Fink, 1996), and the Tcn1/Crz1 transcription factor is directly activated upon dephosphorylation by calcineurin (Matheos et al., 1997; Stathopoulos and Cyert, 1997). However, neither of these targets were necessary for the CCS pathway in yeast (Bonilla et al., 2002). Activated calcineurin was recently found to dephosphorylate the protein kinase Hsl1, causing its delocalization and degradation (Mizunuma et al., 2001). During vegetative growth, Hsl1 inactivates the protein kinase Swe1 (Ma et al., 1996; Tanaka and Nojima, 1996), which otherwise would inhibit the major cyclin-dependent kinase of yeast Cdc28 and block entry into mitosis (Russell et al., 1989; Booher et al., 1993). Activated calcineurin can therefore delay the progression from G2 phase to M phase in the cell division cycle by increasing Swe1-dependent phosphorylation of Cdc28 (Mizunuma et al., 1998). Results presented herein show that ER-damaging agents can induce Swe1-dependent delays at G2/M phase, but surprisingly this response was largely independent of calcineurin. The CCS pathway therefore seems to operate independently of Hsl1, Swe1, and a cell cycle checkpoint that monitors and responds to ER malfunction.
Arrest in G2/M phase occurs in yeast cells that fail to produce a daughter cell, due, for example, to defects in the actin cytoskeleton (Lew and Reed, 1995; McMillan et al., 1998). The arrest is caused by inhibitory phosphorylation of the cyclin-dependent kinase Cdc28 by Swe1 and is reversed by the action of Mih1 protein phosphatase (Russell et al., 1989). Recent findings suggest that G2/M arrest in response to actin-depolymerizing drugs may involve inhibition of Mih1 by the Mpk1 protein kinase (Harrison et al., 2001). Mpk1 is the terminal mitogen-activated protein (MAP) kinase in the cell integrity MAP kinase cascade that is activated in response to cell wall-damaging agents and secretory defects. The transmembrane sensors of these forms of membrane stress are thought to include Mid2 (Ono et al., 1994; Ketela et al., 1999; Rajavel et al., 1999) and Hcs77/Wsc1 (Gray et al., 1997; Verna et al., 1997), which activate Rom2 (Philip and Levin, 2001) and the small GTPase Rho1 (Nonaka et al., 1995; Kamada et al., 1996), which binds and activates Pkc1 (Levin et al., 1990; Watanabe et al., 1994). Pkc1 then activates the MAPKKK Bck1 (Costigan et al., 1992; Lee and Levin, 1992), the MAPKKs Mkk1/2 (Irie et al., 1993), and finally the MAPK Mpk1 (Lee et al., 1993) in a linear signaling pathway. Targets of Mpk1 in this pathway include the transcription factor Rlm1 (Watanabe et al., 1997) as well as the SBF-transcription factor components Swi4 and Swi6 (Madden et al., 1997). Remarkably, the transmembrane sensors may also initiate signaling from within secretory organelles in response to ER damage (Nierras and Warner, 1999; Nanduri and Tartakoff, 2001).
Herein, we screened a collection of protein kinase mutants for defects in coupling ER stress to the CCS pathway and identified Mpk1 and other components of the cell integrity MAP kinase cascade. Mpk1 became activated in response to ER stress and directly or indirectly stimulated the Cch1-Mid1 Ca2+ channel during conditions of ER stress. A variety of agents that cause ER stress also lead to a Swe1-dependent delay or arrest at G2/M. However, neither Mpk1 nor calcineurin was required for G2/M delay in most cells under any conditions. Conversely, Swe1 was not required for execution of the CCS pathway. We propose that MAP kinase signaling toward the Cch1-Mid1 Ca2+ channel is a primary and essential response to ER stress in yeast.
MATERIALS AND METHODS
Strains and Growth Conditions
All yeast strains in this study (Table 1) were derived from either BY4741 (MATa his3-1 leu2-2 met15-0 ura3-0), EG123 (leu2-3112 trp1-1 ura3-52 his4 can1), or W303-1a (MATa ade2-1 can1-100 his3-1 leu2-3112 trp1-1 ura3-1) parent strains. Yeast strains were grown in synthetic complete (SC) medium containing 2% glucose (Sherman et al., 1986) by using reagents from Difco (Detroit, MI) and Sigma-Aldrich (St. Louis, MO). Tunicamycin (Sigma-Aldrich) was dissolved in methanol and added to culture medium at a final concentration of 2.5 μg/ml. The yeast mating pheromone α-factor (U.S. Biochemical, Cleveland, OH), latrunculin B (BIOMOL Research Laboratories, Plymouth Meeting, PA), miconazole (Sigma-Aldrich), and FK506 (Fujisawa Healthcare, Deerfield, IL) were dissolved in dimethyl sulfoxide and used at a final concentrations of 40 μM, 100 μM, 6 μM, and 2 μg/ml, respectively. Chlorpromazine (Sigma-Aldrich) was added to cultures at final concentrations of 250 μM where indicated.
Table 1.
S. cerevisiae strains used in this study
| Strain | Genotype | Source or Reference |
|---|---|---|
| K601 | W303-1a | Wallis et al. (1989) |
| K1257 | BY4741 | Bonilla et al. (2002) |
| K1259 | BY4741 ire1::G418 | Bonilla et al. (2002) |
| RG00993 | BY4741 mpk1::G418 | Research Genetics |
| RG01328 | BY4741 bck1::G418 | Research Genetics |
| RG01137 | BY4741 mck1::G418 | Research Genetics |
| RG04951 | BY4741 hsl1::G418 | Research Genetics |
| RG01238 | BY4741 swe1::G418 | Research Genetics |
| RG02739 | BY4741 rlm1::G418 | Research Genetics |
| RG06109 | BY4741 swi4::G418 | Research Genetics |
| RG04131 | BY4741 swi6::G418 | Research Genetics |
| RG00612 | BY4741 mih1::G418 | Research Genetics |
| RG04847 | BY4741 cch1::G418 | Research Genetics |
| DL1101 | EG123 | Dr. David Levin |
| DL2282 | EG123 mid2::URA3 hcs77::LEU2 | Rajavel et al. (1999) |
| DL376 | EG123 pkc1::LEU2 | Levin and Bartlett-Heubusch (1992) |
SDS-Urea Gradient Gel Electrophoresis and Western Blotting
Cell extracts were prepared from yeast strain ELY242 carrying epitope tagged CCH1-MYC construct as described previously (Locke et al., 2000). Samples were loaded onto 0.75-mm-thick linear SDS gradient gels containing 3–5% acrylamide and 3–8 M urea as described previously (King et al., 1986) and electrophoresed for ∼20 h at 65 mV. Proteins were transferred to polyvinylidene difluoride membrane by electroblotting, and probed with 9E10 anti-MYC monoclonal antibody (mAb) (Santa Cruz Biotechnology, Santa Cruz, CA) plus horseradish peroxidase-conjugated goat anti-mouse antibody (Jackson Immunoresearch Laboratories, West Grove, PA), and visualized using a chemiluminescence ECL kit (Amersham Biosciences, Piscataway, NJ).
In some experiments, Cch1-MYC was immunoprecipitated and treated with protein phosphatases in vitro before electrophoresis and Western blotting. For these experiments, a log-phase culture of strain ELY242 growing in SC medium was treated with 2.5 μg/ml tunicamycin and 2 μg/ml FK506 for 2 h at 30°C. Cells were harvested by centrifugation (40 ml at OD600 = 0.5), washed with ice-cold water, resuspended in BB buffer (0.3 M sorbitol, 0.1 M NaCl, 5 mM MgCl2, 10 mM Tris-HCl,]pH 7.5, protease inhibitors), broken by vortexing with acid-washed glass beads (425–600 μm in diameter, 2 min, 4°C), centrifuged at low speed to remove beads and cell debris, and the supernatant was centrifuged at 135,000 × g for 30 min at 4°C. The crude membrane pellet was resuspended in 3.0 ml of immunoprecipitation buffer (50 mM Tris-HCl, pH 8.0, 1.0% Triton X-100, 150 mM NaCl, 2 mM EDTA, protease inhibitors) and recentrifuged. The clear supernatant was then incubated with 100 μl of mAb 9E10-agarose bead conjugate (500 μg of 9E10/250 μl of agarose; Santa Cruz Biotechnology) for 16 h at 4°C with gentle agitation. Beads were collected by brief centrifugation, washed three times with immunoprecipitation buffer, and two washes with 50 mM Tris-HCl, pH 7.4. Washed beads were resuspended in reaction buffer (50 mM Tris-HCl, pH 7.4, 1 mg/ml bovine serum albumin, 100 μM CaCl2, 1 μM calmodulin [Upstate Biotechnology, Lake Placid, NY], 1 mM NiCl2), split into two equal fractions, and incubated for 1.5 h at 37°C with or without calcineurin (10 U; Sigma-Aldrich). A third equal portion of the washed beads was resuspended in CIP reaction buffer (50 mM Tris-HCl, pH 7.9, 100 mM NaCl, 10 mM MgCl2, 1 mM dithiothreitol, 100 U of alkaline phosphatase, calf intestinal [New England Biolabs, Beverly, MA]) for 1.5 h at 37°C. Beads were then collected by centrifugation, washed two times with 1 ml of 50 mM Tris-HCl, pH 7.4, resuspended in protein sample buffer supplemented with 100 mM dithiothreitol, loaded onto urea gradient gels, and processed for Western blotting as described above.
45Ca2+ Accumulation Measurements
Cell were grown overnight in SC media to log phase at 30°C, and total cellular accumulation of Ca2+ was measured as described previously (Cunningham and Fink, 1996). Briefly, log phase yeast cultures with appropriate treatments were supplemented with tracer quantities of 45CaCl2 (Amersham Biosciences) and incubated for 4 h at 30°C, followed by filtration onto GF/F filters (Whatman, Maidstone, UK). Harvested cells were then washed three times with ice-cold buffer (10 mM CaCl2, 5 mM HEPES-NaOH, pH 6.5), and filters were dried at 90°C for 1 h. Filters were placed in scintillation vials with OptiFluor (PerkinElmer Life Sciences, Boston, MA) scintillation cocktail and counted using a 1600 TR liquid scintillation counter (PerkinElmer Life Sciences). This method was used to screen all the available protein kinase-deficient mutants in yeast (a total of 99 mutants in the BY4741 strain back-ground obtained from Research Genetics (Huntsville, AL) as annotated in the YPD database from Incyte Systems, Palo Alto, CA) for defects in the stimulation of Cch1-Mid1 activity. Only two mutants, bck1 and slt2/mpk1, were significantly defective in Cch1-Mid1 stimulation in response to treatment with tunicamycin plus FK506.
Northern Blot Analysis
HAC1 mRNA levels were measured by Northern blot analysis as described previously (Bonilla et al., 2002). Briefly, log phase cells grown in YPD medium and treated with 2.5 μg/ml tunicamycin for 60 min were chilled on ice, harvested by centrifugation at 700 × g at 4°C for 5 min, and total RNA was extracted as described in Zhao et al., 1998. For Northern blot analysis, 20 μg of total RNA was loaded and electrophoresed through 1% agarose-formaldehyde gel, blotted onto Hybond-N+ membrane by capillary transfer, and RNA was cross-linked to membranes by UV radiation. Membranes were blocked and hybridized for 20 h with [α-32P]dCTP-labeled HAC1 DNA probe (Bonilla et al., 2002), washed, and visualized by autoradiography.
β-Galactosidase Assays
Mpk1 activity was monitored by measuring expression of a plasmid-born MPK1-lacZ reporter gene (Jung et al., 2002). Yeast strains carrying the MPK1-lacZ reporter gene were grown to log phase overnight at 30°C in SC media lacking uracil. Cultures were split and treated with tunicamycin and FK506 for 4 h at 30°C as indicated. Cells were then harvested by centrifugation, permeabilized with SDS and chloroform, and assayed for β-galactosidase activity by using the colorimetric substrate O-nitrophenyl β-d-galactopyranoside (Sigma-Aldrich) as described previously (Guarente, 1983).
Immune Complex Kinase Assays
MPK1-HA carried on a high dosage YEp352 plasmid (Kamada et al., 1995) was introduced into wild-type strain DL1101. To induce Mpk1 activity by heat shock, cultures grown at 23°C were diluted 1:1 with media preheated to 55°C, and the resulting culture was placed at 39°C for 30 min before harvesting. Alternatively, cultures were mock-treated by 1:1 dilution into 23°C media, and the resulting culture was maintained at 23°C for 30 min before harvesting. Cultures were also treated with chlorpromazine for 120 min or tunicamycin for various amounts of time. Cells were then harvested, lysed, and then Mpk1 proteins were isolated by immunoprecipitation and assayed for protein kinase activity using myelin basic protein (Sigma-Aldrich) as described previously (Kamada et al., 1995). Aliquots of extracts prepared for immunecomplex kinase assays were also separated by SDS-PAGE by using 8% acrylamide gels, electroblotted to polyvinylidene difluoride membranes, and probed with 12CA5 anti-hemagglutinin (HA) mAb (Roche Diagnostics, Indianapolis, IN) plus horseradish peroxidase-conjugated goat anti-mouse secondary antibody (Jackson Immunoresearch Laboratories) and visualized by chemiluminescence as described above.
Microscopic Assays for Cell Death and Swe1-dependent G2/M Delay
Dead cells were stained with 100 μg/ml methylene blue (Sigma-Aldrich) and counted microscopically as described previously (Bonilla et al., 2002). At least 200 live and dead cells were counted at each time point.
Swe1-dependent G2/M delay was measured microscopically on asynchronous populations of cells essentially as described previously (McMillan et al., 1998). Cultures were grown to log-phase in SC medium at 30°C, split, and treated with tunicamycin and/or FK506. After various times of incubation with the drugs, cells were fixed in 70% ethanol for 14 h at 4°C, harvested by centrifugation at 4000 × g for 5 min, stained with 0.4 μg/ml 4′,6′-diamidino-2-phenylindole (Sigma-Aldrich), and viewed on an Axioplan microscope (Carl Zeiss, Thornwood, NY) equipped with epifluorescence illumination. At each time point, 200 unbudded cells were scored as mononucleate or multinucleate. Similar results were obtained using cultures that had been presynchronized in G1 phase by brief treatment with α-factor mating pheromone. However, mpk1 mutants could not be analyzed by this method due to rapid cell death.
RESULTS
Cch1-Mid1 Stimulation Requires Mpk1 and Components of the Cell Integrity MAP Kinase Cascade
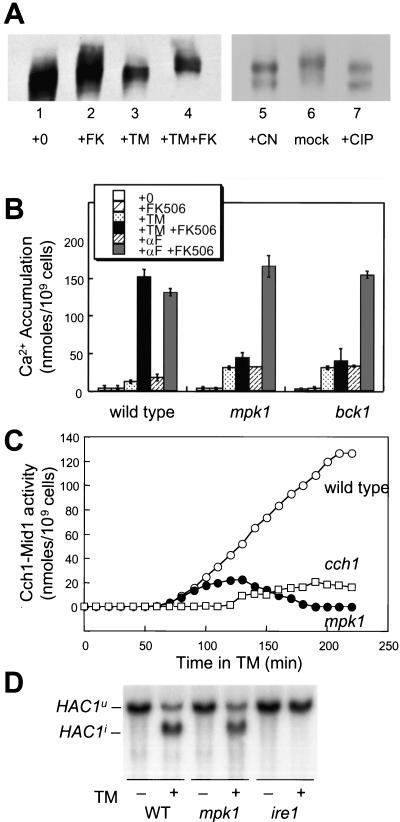
The Cch1-Mid1 channel seems to be inhibited by the protein phosphatase calcineurin during activating conditions such as ER stress (Locke et al., 2000; Muller et al., 2001; Bonilla et al., 2002). To investigate the mechanism of Cch1-Mid1 activation and inhibition, the mobility of epitope-tagged Cch1-MYC on SDS-urea gradient gels was monitored in different growth conditions. The 240-kDa Cch1-MYC protein extracted from cells growing in standard medium migrated as a series of bands that became more compact after 2-h incubation with tunicamycin (Figure 1A). Inhibition of calcineurin with the drug FK506 during the tunicamycin treatment resulted in a large mobility shift (lane 4) that was mimicked in a calcineurin-deficient cnb1 mutant (our unpublished data). Similar mobility shifts were seen in vitro after incubation of immunoprecipitated Cch1-MYC with either purified calcineurin or purified calf intestinal phosphatase (lanes 5–7). Thus, Cch1 can be directly dephosphorylated by calcineurin in vitro and a similar reaction in vivo may inhibit the activity of the Cch1-Mid1 Ca2+ channel.
Figure 1.
Identification of kinases required for Cch1-Mid1 Ca2+ channel activation. (A) SDS-urea gradient gel analysis of Cch1-MYC extracted from wild-type cells (strain K1257) treated with tunicamycin (2.5 μg/ml) and FK506 (2.0 μg/ml) as indicated (lanes 1–4). In the right panel (lanes 5–7), Cch1-MYC was first immunoprecipitated from cells grown with tunicamycin and FK506 as described above and treated in vitro with either purified calcineurin, reaction buffer lacking calcineurin, or purified calf intestinal phosphatase. (B) 45Ca2+ accumulation in wild-type cells, mpk1 mutants, and bck1 mutants (strains K1257, RG00993, and RG01328, respectively) was measured after growth for 4 h in SC medium supplemented with tunicamycin, α-factor mating pheromone, and FK506 as indicated. The average of three independent experiments (± SD) is shown. (C) Accumulation of 45Ca2+ in wild-type cells (open circles), cch1 mutants (open squares), and mpk1 mutants (closed circles) (strains K1257, RG04847, and RG00993, respectively) was measured as in A at various times after treatment with tunicamycin plus FK506 and tunicamycin alone. Cch1-dependent Ca2+ influx activity was calculated as the difference between these two measurements at every time point. (D) Northern blot analysis of HAC1 mRNA isolated from wild-type cells, mpk1 mutants, and ire1 mutants (strains K1257, RG00993, and K1259) mutant cells treated with or without tunicamycin for 1 h.
To identify protein kinases involved in the stimulation of Cch1-Mid1 activity, a collection of 99 gene knockout mutants each lacking a different nonessential protein kinase was screened directly for abnormal 45Ca2+ accumulation after treatment with tunicamycin, FK506, or both drugs. In the initial screening, mpk1 and bck1 mutants were found to be required for Cch1-Mid1 activation by tunicamycin plus FK506. These mutations abolish different steps of the cell integrity MAP kinase pathway (Torres et al., 1991; Lee and Levin, 1992; Lee et al., 1993). In response to tunicamycin, the mpk1 and bck1 null mutants exhibited a small increase in 45Ca2+ accumulation, but like cch1 and mid1 mutants (Bonilla et al., 2002), there was little or no enhancement upon treatment with FK506 (Figure 1B). In response to a control stimulus, the α-factor mating pheromone, stimulation and inhibition of Cch1-Mid1 activity were not altered in mpk1 and bck1 mutants relative to wild-type (Figure 1B). Mpk1 and Bck1 activity was also required for stimulation of Cch1-Mid1 activity in response to dithiothreitol, an inhibitor of disulfide bonding in the ER, and miconazole, an inhibitor of ergosterol biosynthesis in the ER (our unpublished data). Thus, the MAP kinase Mpk1 and its upstream regulator Bck1 were specifically required for the stimulation of Cch1-Mid1 activity in response to ER stresses but not in response to pheromone signaling.
To examine the involvement of Mpk1 more carefully, 45Ca2+ accumulation into wild-type and mpk1 mutants was measured at various times after addition of tunicamycin or tunicamycin plus FK506. The difference between these two measurements largely reflects Cch1-Mid1 activity as indicated by the strong Ca2+ accumulation in wild-type cells but not in cch1 mutants (Figure 1C). In mpk1 mutants, Cch1-Mid1 activity seemed similar to wild-type at early time points but eventually declined to low levels (Figure 1C, closed circles). To rule out the possibility that mpk1 mutants had become resistant or unresponsive to ER stress, activation of the UPR signaling pathway was monitored by Northern blot analysis of HAC1 pre mRNA splicing. Splicing of the HAC1u pre mRNA to the mature HAC1i mRNA occurred with equal kinetics in wild-type and mpk1 mutants after treatment with tunicamycin (Figure 1D). Together with the finding that calcineurin regulation of the Tcn1/Crz1 transcription factor occurs normally in mpk1 mutants (our unpublished data), we conclude that Mpk1 and Bck1 are required for maximal stimulation of the Cch1-Mid1 Ca2+ channel specifically in response to ER stress.
Stimulation of Cch1-Mid1 Activity Is Dependent on Activation of Mpk1 but Not Its Known Targets
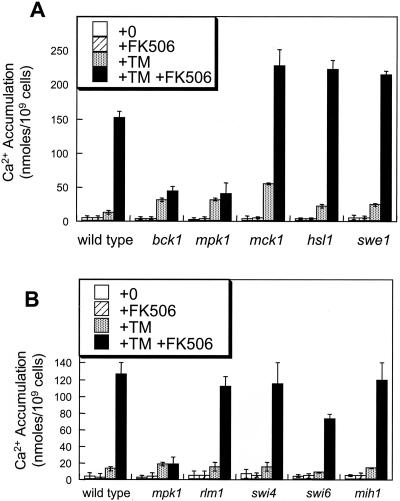
Mpk1 may directly or indirectly affect the activities of several other nonessential protein kinases, including Mck1, Hsl1 (Mizunuma et al., 1998), and Swe1 (Harrison et al., 2001). However, none of these putative downstream kinases were required for Cch1-Mid1 activation by tunicamycin plus FK506 (Figure 2A). The mck1 mutant exhibited a wild-type response to tunicamycin plus FK506, but exhibited abnormally high 45Ca2+ accumulation in response to tunicamycin without FK506 (Figure 2A). This observation is consistent with a partial loss of calcineurin activity in mck1 mutants (Gallagher, Hilioti, Low-Nam, Ramaswamy, Gajer, Kingsbury, Birchwood, Levchenko, and Cunningham, unpublished data). Mpk1 is thought to regulate the activities of several other factors, including the Rlm1 transcription factor (Watanabe et al., 1997), the Swi4–Swi6 transcription factor complex (Madden et al., 1997), and the Mih1 protein phosphatase (Harrison et al., 2001). However, rlm1, swi4, swi6, and mih1 null mutants all exhibited wild-type patterns of Ca2+ accumulation in response to tunicamycin and FK506 (Figure 2B). Therefore, none of these Mpk1 targets were required for stimulation of Cch1-Mid1 activity.
Figure 2.
Mpk1 stimulates Cch1-Mid1 activity independently of other known targets. (A) 45Ca2+ accumulation was measured in wild-type cells, bck1 mutants, mpk1 mutants, mck1 mutants, hsl1 mutants, and swe1 mutants as described in Figure 1B. (B) 45Ca2+ accumulation in wild-type cells, mpk1 mutants, rlm1 mutants, swi4 mutants, swi6 mutants, and mih1 mutants was measured as in Figure 1B.
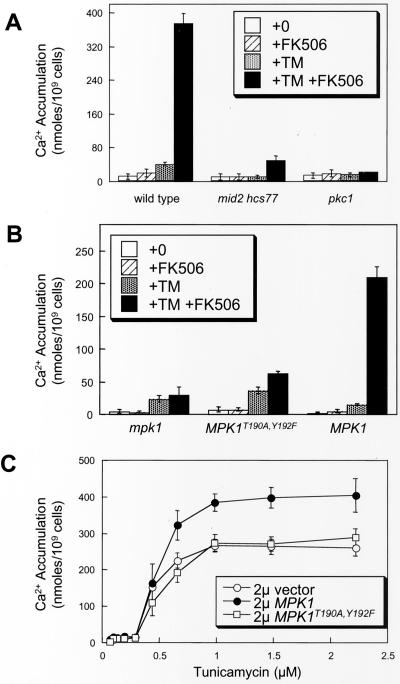
Several factors upstream of Mpk1 in the cell integrity MAP kinase cascade were examined for stimulation of Cch1-Mid1 activity. Ca2+ accumulation was measured in pkc1 null mutants, mid2 hcs77 double mutants, and isogenic wild-type strain EG123 in medium containing 0.5 M sorbitol as an osmotic stabilizer to maintain viability. Stimulation of Cch1-Mid1 activity by tunicamycin plus FK506 treatment was abolished in both pkc1 mutants and mid2 hcs77 double mutants (Figure 3A). Thus, the entire cell integrity MAP kinase cascade from transmembrane sensors to Mpk1 seemed to be required for stimulation of Cch1-Mid1 activity in response to ER stress.
Figure 3.
Mpk1-induced Ca2+ influx through Cch1 requires other components of the cell integrity pathway. (A) 45Ca2+ accumulation was measured in wild-type cells, mid2 hcs77 double mutants, and pkc1 mutants (strains DL1101, DL2282, and DL376) was measured as in Figure 1B except that SC media were supplemented with 0.5 M sorbitol for osmotic support. (B) 45Ca2+ accumulation was measured in mpk1 mutant cells (strain RG00993) transformed with high-dosage plasmids carrying MPK1-HA, mpk1T190A, Y192F-HA, or vector control (pRS425) as in Figure 1B except that cells were grown in SC medium lacking leucine. (C) 45Ca2+ accumulation was measured in wild-type cells (strain K601) transformed with the same plasmids as in B after incubation FK506 and varying concentrations of tunicamycin.
We next investigated whether phosphorylation-dependent Mpk1 activation was necessary for stimulation of Cch1-Mid1 activity by measuring Ca2+ accumulation in cells expressing only a nonphosphorylatable form of Mpk1. Mpk1 becomes activated upon phosphorylation of Thr-190 and Tyr-192 by the MAP kinase kinases Mkk1 and Mkk2 (Lee et al., 1993). Cells expressing only MPK1T190A,Y192F in which Thr-190 and Tyr-192 were substituted for Ala and Phe were not able to activate Cch1-Mid1 in response to tunicamycin plus FK506 (Figure 3B). Overexpression of MPK1T190A,Y192F in wild-type cells did not affect Cch1-Mid1 activity with or without tunicamycin treatment (Figure 3C). Overexpression of wild-type MPK1 increased Cch1-Mid1 activity after treatment with tunicamycin plus FK506 but not with FK506 alone (Figure 3C; our unpublished data). These results suggest that activation of Mpk1 through Pkc1, Bck1, and Mkk1/Mkk2 is necessary for stimulation of Cch1-Mid1 activity in response to ER stress.
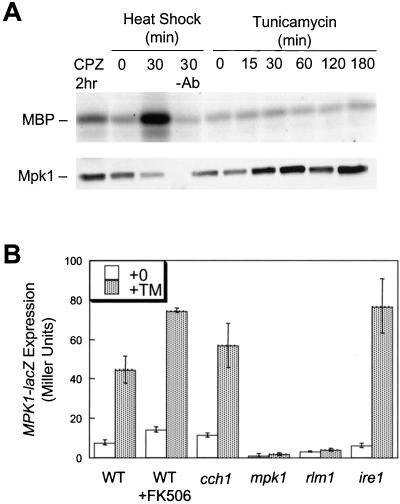
The extent of Mpk1 activation in response to tunicamycin treatment was directly measured in vitro by using immune complex kinase assays. A functional epitope-tagged Mpk1-HA variant was immunoprecipitated from yeast cell extracts and assayed for phosphorylation of myelin basic protein as substrate (Kamada et al., 1995). We first confirmed previous reports that Mpk1 activity can be strongly stimulated by heat shock or membrane stress caused by treatment with chlorpromazine (Figure 4A, lanes 2–4). Wild-type cells treated with tunicamycin for various times up to 3 h exhibited no significant increase in Mpk1 kinase activity over background (lanes 5–10). Inclusion of FK506 during the tunicamycin treatment also failed to stimulate Mpk1 activity into the detectable range (our unpublished data).
Figure 4.
Mpk1 activity is stimulated by tunicamycin treatment independently of Ire1 and Ca2+. (A) Immune complex kinase assays were performed using Mpk1-HA isolated from wild-type cells (strain DL1101 transformed with YEp352 MPK1-HA) that had been grown at 23°C and either treated with 250 μM chlorpromazine for 2 h (lane 1), mock-shifted to 23°C (lane 2), heat shocked at 39°C for 30 min (lane 3), or treated with 2.5 μg/ml tunicamycin for the indicated times (lanes 5–10). Immunoprecipitates were also analyzed by SDS-PAGE Western blotting for immunodetection of Mpk1-HA (bottom). (B) Wild-type cells, cch1 mutants, mpk1 mutants, rlm1 mutants, and ire1 mutants (strains K1257, RG04847, RG00993, RG02739, and K1259) bearing the MPK1-lacZ reporter gene were grown to log phase in SC medium lacking uracil and treated with tunicamycin with or without FK506. After 4 h, the cells were harvested and assayed for β-galactosidase activity. The mean of three independent transformants is shown (± SD).
The in vitro kinase assays may not be sensitive enough to detect a small or localized activation of Mpk1 in response to tunicamycin treatment, so the expression of MPK1-lacZ, an Mpk1- and Rlm1-dependent reporter gene (Jung et al., 2002), was monitored in response to ER stress. Tunicamycin treatment stimulated MPK1-lacZ expression in wild-type cells approximately fourfold above basal levels but had no stimulatory effect in mpk1 or rlm1 mutants (Figure 4B). An ire1 mutant defective in UPR signaling displayed a wild-type response to tunicamycin (Figure 4B). MPK1-lacZ expression was therefore insensitive to UPR signaling but still induced by tunicamycin treatment in an Mpk1- and Rlm1-dependent manner. FK506 had little effect on MPK1-lacZ induction by tunicamycin in either wild-type cells or cch1 mutant cells (Figure 4B), suggesting that calcineurin and Ca2+ influx via Cch1-Mid1 have little or no impact on Mpk1 signaling. Finally, treatment of cells with bis-(o-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid, a Ca2+ chelator, had no inhibitory effect on MPK1-lacZ induction in wild-type and cch1 mutants (our unpublished data). Collectively, these data suggest that Mpk1 activity is significantly stimulated in cells treated with tunicamycin and stimulation is independent of Ca2+, calcineurin signaling, and UPR signaling.
Tunicamycin Treatment Triggers Swe1-dependent Delay in G2/M Phase of the Cell Division Cycle
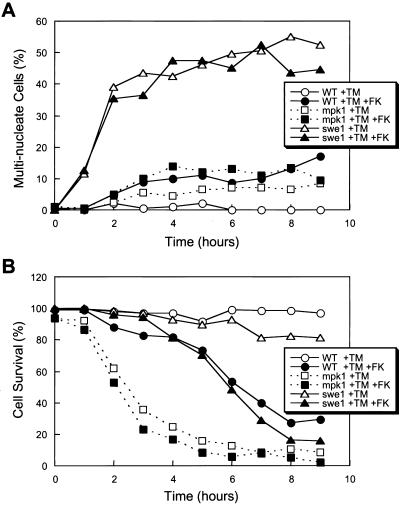
Some yeast strains arrest reversibly after treatment with tunicamycin as unbudded cells containing a single nucleus with replicated DNA (Vai et al., 1987). This response to tunicamycin is very similar to the effects of latrunculin B treatment, which disrupts the actin cytoskeleton, prevents bud emergence, and triggers a Swe1-dependent delay in G2/M phase of the cell cycle (Lew and Reed, 1995; McMillan et al., 1999). To demonstrate that ER stress causes a Swe1-dependent delay or arrest at G2/M in our conditions, asynchronous cultures of wild-type and swe1 mutant cells were treated with tunicamycin or tunicamycin plus FK506 and assayed microscopically for the appearance of abnormal cells containing two or more nuclei. In striking contrast to wild-type cells (which very rarely became binucleated), >60% of swe1 mutant cells became binucleated or multi-nucleated after tunicamycin treatment (Figure 5A). The addition of FK506 in combination with tunicamycin slightly increased the occurrence of multi-nucleated cells to only 10% in both wild-type and swe1 mutants (Figure 5A). Similar results were obtained using synchronized cultures prepared by prior brief treatment with α-factor (our unpublished data). The results show that tunicamycin treatment causes a Swe1-dependent delay in nuclear division similar to the G2/M delay observed in response to latrunculin B. The tunicamycin-induced delay seemed to be largely independent of calcineurin as judged by the mild effects of FK506 (Figure 5A).
Figure 5.
Tunicamycin induces Swe1-dependent delay at G2/M phase of the cell cycle and Swe1-independent cell survival pathway (A) Asynchronous log-phase cultures of wild-type cells, mpk1 mutants, and swe1 mutants (strains K1257, RG00993, and RG01238) were treated with tunicamycin or tunicamycin plus FK506 for the indicated periods of time, fixed, stained with DAPI, and analyzed by fluorescence microscopy. The percentage of cells in the population containing two or more nuclei is plotted. (B) Dead cells in the cultures used in A were identified by light microscopy after staining with methylene blue, and the percentage of surviving cells in the population is plotted.
Failure to arrest in G2/M phase is lethal to cells treated with latrunculin B (McMillan et al., 1999). To test whether G2/M arrest is necessary for survival of cells undergoing ER stress, the frequency of dead cells in the population was determined microscopically after treatment with tunicamycin, FK506, and other drugs. Wild-type and swe1 mutant cells remained highly viable for long periods of time after treatment with tunicamycin but died rapidly after treatment with tunicamycin plus FK506 (Figure 5B). Survival of mpk1 mutant cells in tunicamycin was severely compromised with or without FK506 (Figure 5B) and was not suppressed by the addition of sorbitol as an osmotic stabilizer (our unpublished data). Thus, execution of the Swe1-dependent delay in the G2/M phase of the cell cycle was not essential for survival of cells undergoing ER stress but the activation of Mpk1 and calcineurin was critical for long-term survival of these cells.
The CCS Pathway Does Not Rely on G2/M Delay
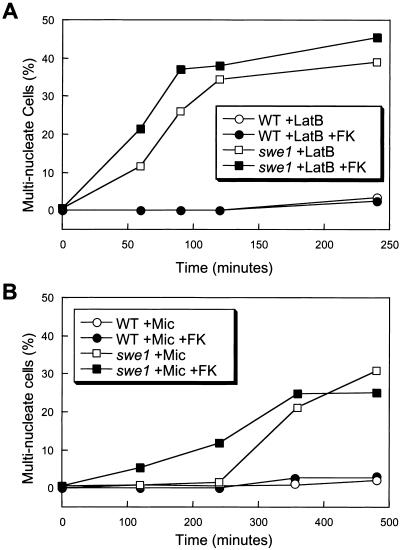
The findings described above show that calcineurin is not required for Swe1-dependent G2/M delay in most cells treated with tunicamycin. In other conditions (high calcium stress), calcineurin activation seemed to cause a G2/M delay by indirect activation of Swe1 (Mizunuma et al., 1998; Mizunuma et al., 2001). The role of calcineurin in the classical G2/M delay triggered by actin perturbation has not been established. Wild-type cells treated with latrunculin B or latrunculin B plus FK506 did not become binucleated (Figure 6A) and remained viable for extended periods of time (our unpublished data). In contrast, most swe1 mutant cells rapidly became binucleated after treatment with latrunculin B and inclusion of FK506 modestly accelerated the rate of their appearance (Figure 6A). Therefore, calcineurin signaling was not necessary for the Swe1-dependent G2/M arrest during the response to either actin depolymerization or ER stress although calcineurin may delay G2/M in other conditions (Mizunuma et al., 2001).
Figure 6.
Latrunculin B and miconazole trigger the morphogenesis checkpoint with different kinetics. The percentage of multinucleated cells in the population of wild-type and swe1 mutant cultures (strains K1257 and RG01238) is determined as in Figure 5A after treatment with latrunculin B (A) or miconazole (B) with or without K506.
Azole-class antifungal drugs inhibit an enzyme in the ER required for the biosynthesis of ergosterol and this form of ER stress slows cell growth and activates the CCS pathway in yeast (Bonilla et al., 2002). Interestingly, the azole drug miconazole induced the accumulation of bi- and multinucleated cells in populations of swe1 mutants but not wild-type cells (Figure 6B). The relatively slow appearance of binucleated cells in swe1 mutants was slightly enhanced in the presence of FK506 (Figure 6B). Survival of cells treated with miconazole was highly sensitive to FK506 (Bonilla et al., 2002), but also independent of Swe1 (our unpublished data). Thus, miconazole treatment caused a mild Swe1-dependent G2/M delay that was independent of calcineurin and this delay seemed to be far less important than calcineurin in preventing cell death.
To summarize these findings, cells treated with latrunculin B required Swe1, but not calcineurin, for G2/M arrest and long-term survival. Cells treated with tunicamycin or miconazole required calcineurin for long-term survival but did not require Swe1 for survival even though a Swe1-dependent G2/M delay was induced. Although calcineurin was proposed to regulate Swe1 in high Ca2+ conditions (Mizunuma et al., 1998, 2001), we found little or no evidence linking calcineurin to Swe1 function in response to the three inhibitors used herein. Therefore, the CCS pathway and the mechanism responsible for G2/M delay seem to operate independently in most conditions.
DISCUSSION
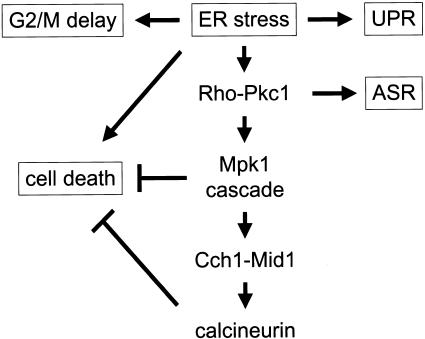
The Cch1-Mid1 high-affinity Ca2+ channel in yeast becomes activated in response to diverse stimuli, including mating pheromones (Iida et al., 1994; Fischer et al., 1997; Paidhungat and Garrett, 1997; Muller et al., 2001) and mutations or drugs that affect enzymes in the ER (Bonilla et al., 2002). In both situations, Cch1-Mid1 activity is strongly increased by the loss of calcineurin, as if this protein phosphatase were inhibiting Cch1-Mid1 activation by a negative feedback mechanism (Muller et al., 2001; Bonilla et al., 2002). Herein, we show that Cch1 is phosphorylated in vivo and dephosphorylated by calcineurin in vitro and probably in vivo. We also identify the cell integrity MAP kinase Mpk1 as a direct or indirect activator of Cch1-Mid1 in response to ER stress. Although ER stress seems to cause a Swe1-dependent delay in G2 phase of the cell division cycle, this delay was independent of calcineurin and also not required for calcineurin-dependent prevention of cell death. A model of these interactions is depicted in Figure 7.
Figure 7.
Model of the cellular responses to ER stresses in yeast. ER stress can induce the UPR involving Ire1 (1), cell cycle delay at G2/M involving Swe1 (2), a cell death pathway (3), and the cell integrity MAP kinase cascade involving Pkc1 and Mpk1, which stimulates the Cch1-Mid1 Ca2+ channel and calcineurin signaling (4). Calcineurin and Mpk1 also antagonize cell death and Pkc1 regulates transcription of genes encoding ribosomal subunits and other phenomena known as the arrest of secretion response (ASR).
The Pkc1-Mpk1 Cell Integrity MAP Kinase Cascade Can Activate the Cch1-Mid1 Ca2+ Channel and Calcineurin
The cell integrity MAP kinase cascade is thought to monitor stress in the plasma membrane arising through such conditions as defects in the cell wall, hypotonic shock, heat shock, intercalation of hydrophobic molecules, and mutations that block protein trafficking in the secretory pathway. In all of these cases, Mpk1 is known to be activated by a cascade of upstream signaling factors that include homologs of MAPKK (Mkk1 and Mkk2), MAPKKK (Bck1), protein kinase C (Pkc1), rho-type small GTPase (Rho1), GDP/GTP exchange factor (Rom2), and transmembrane receptors (Wsc1-4). We find that these factors were required for full stimulation of the Cch1-Mid1 Ca2+ channel in the plasma membrane. All these factors seem to be necessary for the stimulation of Cch1-Mid1 activity in the plasma membrane. However, the source and nature of the activating stimulus are not identified in this study and therefore signaling may originate from the plasma membrane or endomembrane compartments as suggested previously (Li et al., 2000; Nanduri and Tartakoff, 2001). Mpk1 activity significantly increased in cells treated with tunicamycin (Figure 4B) as suggested previously by studies of Mpk1 phosphorylation by upstream kinases Mkk1/Mkk2 (Li et al., 2000; Torres et al., 2002). In contrast to previous assertions (Mizunuma et al., 1998), we found no evidence for activation of Mpk1 by elevated cytosolic free Ca2+ concentrations. No significant effects of Ca2+ depletion, Cch1 mutation, and/or calcineurin inhibition on Mpk1 signaling were detected in our conditions. Therefore, the cell integrity MAP kinase pathway acts upstream of the Cch1-Mid1 Ca2+ channel in response to ER stress.
How do Mpk1 and calcineurin regulate Cch1-Mid1 activity? The simplest hypothesis is that they antagonistically modify the same target(s). We ruled out many of the known targets of Mpk1, including the transcription factors Rlm1 and Swi4-Swi6 and several other regulatory factors Hsl1, Swe1, Mck1, and Mih1, as regulators of Cch1-Mid1 during ER stress. Cch1-Mid1 may therefore constitute another independent target of Mpk1 signaling. Cch1 was phosphorylated in vivo particularly after inhibition of calcineurin and activation of Mpk1 with tunicamycin. As detected by shifts in gel mobility, Cch1 phosphorylation was only partially dependent on Mpk1 but totally dependent on calcineurin (our unpublished data; Figure 1). In mammalian cells, calcineurin can directly regulate many of the Cch1 homologs (Yakel, 1997). Additional experiments will be necessary to determine the precise sites of Cch1 phosphorylation and dephosphorylation and whether these modifications are sufficient to control the Ca2+ influx activity of Cch1-Mid1.
The unfolded protein response in yeast, involving the Ire1 transmembrane receptor/transducer and the Hac1 transcription factor (Patil and Walter, 2001), primarily serves to coordinate gene expression. Activation of the Pkc1-Mpk1 MAP kinase cascade and the calcium signaling pathway in response to ER stress is completely independent of Ire1 and evokes a variety of responses at different branches of the signaling network. For example, one branch emanating from Pkc1 but independent of the Bck1-Mkk1/2-Mpk1 module is responsible for transcriptional repression of many genes involved in protein synthesis (e.g., ribosomal subunits and tRNA) and relocalization of certain nuclear and nucleolar proteins (Li et al., 2000; Nanduri and Tartakoff, 2001). Targets of the Mpk1-dependent transcription factor Rlm1 (Jung and Levin, 1999) and the calcineurin-dependent transcription factor Tcn1/Crz1 (Yoshimoto et al., 2002) are also highly induced independent of Ire1 in response to ER stress (Travers et al., 2000). Independent of these targets, Mpk1 and calcineurin also promote survival and prevent the death of yeast cells undergoing ER stress, and this branch may be responsible for resistance of pathogenic fungi to antifungal drugs (Bonilla et al., 2002).
Mpk1 and Calcineurin in Cell Death and Cell Cycle Control
The recently identified calcineurin target Hsl1 (Mizunuma et al., 1998; Mizunuma et al., 2001) was not necessary for calcineurin-dependent cell survival (our unpublished data). Hsl1 was not required for efficient regulation of the Tcn1/Crz1 transcription factor, the Cch1-Mid1 Ca2+ channel, or the Vcx1 vacuolar H+/Ca2+ exchanger by calcineurin (our unpublished data). Therefore, Hsl1 and another calcineurin-dependent mechanism that prevents cell death in yeast seem to be independent outputs of calcineurin signaling. Because calcineurin is not essential for viability of yeast in non-stressed conditions, our findings suggest that ER stress agents generate a prodeath signal that is counteracted in wild-type cells by the antideath effects of Mpk1 and calcineurin.
While investigating the interactions between Hsl1, Swe1, and calcineurin, we noticed that both tunicamycin and miconazole strongly activated a Swe1-dependent delay at G2/M, which is consistent with previous observations of tunicamycin-treated yeast (Vai et al., 1987). Although calcineurin activation itself can cause a similar G2/M delay in certain conditions (Mizunuma et al., 1998, 2001), our data indicate that calcineurin plays little or no role in the G2/M delay induced by ER stress agents or latrunculin B, a drug that induces G2/M delay as effectively as tunicamycin. In swe1 mutants, FK506 slightly enhanced the failure rate of G2/M arrest (Figure 6), as if calcineurin were regulating a minor Swe1-independent pathway of cell cycle control. Nevertheless, ER stress triggered Swe1-dependent G2/M delay independent of calcineurin in the majority of cells.
An alternative pathway for inducing Swe1-dependent G2/M delay has recently been attributed to Mpk1 activation (Harrison et al., 2001). Mutants lacking Mpk1 became highly binucleated during incubation with latrunculin B, and this effect depended on the function of Mih1, the yeast homolog of Cdc25 phosphatases that is responsible for reversing the inhibitory phosphorylation of Cdc28 by Swe1. Thus, Mpk1 may directly or indirectly inhibit Mih1 and increase the lifetime of Swe1-phosphorylated Cdc28. In response to tunicamycin or miconazole, however, only a small percentage of cells depended on Mpk1 for G2/M delay, and these cells were most likely the ones that used the minor calcineurin-dependent Swe1-independent pathway. One complication in interpreting the relatively minor contribution of Mpk1 to G2/M delay, however, is the very rapid death of mpk1 mutants in these experiments that may have prevented the appearance of binucleated cells.
The findings reported herein suggest that cell cycle delay at G2/M and cell survival mechanisms operate mostly independent of one another in response to membrane stresses. Swe1 was crucial for G2/M delay in most cells, but dispensable for prolonged cell survival. Conversely, Mpk1 and calcineurin were crucial for cell survival but not for Swe1-dependent G2/M delay. That mpk1 mutants treated with FK506 and tunicamycin died more rapidly than wild-type cells under the same conditions indicates Mpk1 can promote cell survival by mechanisms that are independent of calcineurin. These findings not only clarify the relationships between Mpk1, calcineurin, and cell cycle control factors but add new insights into the mechanism by which fungal cells respond and adapt to azole-class antifungal drugs. Fungal-specific inhibitors of the cell integrity MAP kinase cascade, the Cch1-Mid1 Ca2+ channel, or calcineurin (Cruz et al., 2000) may therefore improve the efficacy of azole-class antifungal drugs by blocking resistance and stimulating cell death.
The ER stress-induced G2/M delay in yeast resembles the G2/M delay in mammalian cells that is triggered by inhibitors of Golgi matrix proteins (Sutterlin et al., 2002). The relationship between this phenomenon in animal cells and the strong G2/M delay of yeast cells treated with inhibitors of N-glycosylation in the ER or lacking certain Golgi-localized glycosylation enzymes (Mondesert and Reed, 1996) remains to be established. The ER stress-induced death of yeast cells seems to be preceded by genome fragmentation, phosphatidylserine mislocalization, and other hallmarks of animal apoptosis (Bonilla, preliminary observations). Apoptosis-like death of yeast cells has now been documented under a variety of conditions (Madeo et al., 2002), and therefore a deeper understanding of these processes in yeast may shed light on the mechanisms governing cell proliferation and cell death in mammalian cells.
Acknowledgments
We thank Dr. David Levin and Martin Romeo for strains, plasmids, and technical advice. We also thank Christian Martin for superb technical assistance. We are grateful to Fujisawa Healthcare, Inc., Deerfield, IL for generous gifts of FK506. This work was supported by grants from the DuPont Life Sciences (Young Professors Award) and the National Institutes of Health (R01 GM-053082).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–02–0113. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-02-0113.
References
- Bonilla, M., Nastase, K.K., and Cunningham, K.W. (2002). Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 21, 2343–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booher, R.N., Deshaies, R.J., and Kirschner, M.W. (1993). Properties of Saccharomyces cerevisiae wee1 and its differential regulation of p34CDC28 in response to G1 and G2 cyclins. EMBO J. 12, 3417–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan, C., Gehrung, S., and Snyder, M. (1992). A synthetic lethal screen identifies SLK1, a novel protein kinase homolog implicated in yeast cell morphogenesis and cell growth. Mol. Cell. Biol. 12, 1162–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz, M.C., Del Poeta, M., Wang, P., Wenger, R., Zenke, G., Quesniaux, V.F., Movva, N.R., Perfect, J.R., Cardenas, M.E., and Heitman, J. (2000). Immunosuppressive and nonimmunosuppressive cyclosporine analogs are toxic to the opportunistic fungal pathogen Cryptococcus neoformans via cyclophilin-dependent inhibition of calcineurin. Antimicrob. Agents Chemother. 44, 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz, M.C., Goldstein, A.L., Blankenship, J.R., Del Poeta, M., Davis, D., Cardenas, M.E., Perfect, J.R., McCusker, J.H., and Heitman, J. (2002). Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21, 546–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, K.W., and Fink, G.R. (1996). Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in yeast. Mol. Cell. Biol. 16, 2226–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Poeta, M., Cruz, M.C., Cardenas, M.E., Perfect, J.R., and Heitman, J. (2000). Synergistic antifungal activities of bafilomycin A1, fluconazole, and the pneumocandin MK-0991/caspofungin acetate (L-743,873) with calcineurin inhibitors FK506 and L-685, 818 against Cryptococcus neoformans. Antimicrob Agents Chemother. 44, 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, M., Schnell, N., Chattaway, J., Davies, P., Dixon, G., and Sanders, D. (1997). The Saccharomyces cerevisiae CCH1 gene is involved in calcium influx and mating, FEBS Lett. 419, 259–262. [DOI] [PubMed] [Google Scholar]
- Gray, J.V., Ogas, J.P., Kamada, Y., Stone, M., Levin, D.E., and Herskowitz, I. (1997). A role for the Pkc1 MAP kinase pathway of Saccharomyces cerevisiae in bud emergence and identification of a putative upstream regulator, EMBO J. 16, 4924–4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente, L. (1983). Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 101, 181–191. [DOI] [PubMed] [Google Scholar]
- Harrison, J.C., Bardes, E.S., Ohya, Y., and Lew, D.J. (2001). A role for the Pkc1p/Mpk1p kinase cascade in the morphogenesis checkpoint. Nat. Cell Biol. 3, 417–420. [DOI] [PubMed] [Google Scholar]
- Iida, H., Nakamura, H., Ono, T., Okumura, M.S., and Anraku, Y. (1994). MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol. Cell. Biol. 14, 8259–8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie, K., Takase, M., Lee, K.S., Levin, D.E., Araki, H., Matsumoto, K., and Oshima, Y. (1993). MKK1 and MKK2, which encode Saccharomyces cerevisiae mitogen-activated protein kinase-kinase homologs, function in the pathway mediated by protein kinase C. Mol. Cell. Biol. 13, 3076–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, U.S., and Levin, D.E. (1999). Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 34, 1049–1057. [DOI] [PubMed] [Google Scholar]
- Jung, U.S., Sobering, A.K., Romeo, M.J., and Levin, D.E. (2002). Regulation of the yeast Rlm1 transcription factor by the Mpk1 cell wall integrity MAP kinase. Mol. Microbiol. 46, 781–789. [DOI] [PubMed] [Google Scholar]
- Kamada, Y., Jung, U.S., Piotrowski, J., and Levin, D.E. (1995). The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9, 1559–1571. [DOI] [PubMed] [Google Scholar]
- Kamada, Y., Qadota, H., Python, C.P., Anraku, Y., Ohya, Y., and Levin, D.E. (1996). Activation of yeast protein kinase C by Rho1 GTPase. J. Biol. Chem. 271, 9193–9196. [DOI] [PubMed] [Google Scholar]
- Ketela, T., Green, R., and Bussey, H. (1999). Saccharomyces cerevisiae Mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway. J. Bacteriol. 181, 3330–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, S.M., Otter, T., and Witman, G.B. (1986). Purification and characterization of Chlamydomonas flagellar dyneins. Methods Enzymol. 134, 291–306. [DOI] [PubMed] [Google Scholar]
- Lee, K.S., Irie, K., Gotoh, Y., Watanabe, Y., Araki, H., Nishida, E., Matsumoto, K., and Levin, D.E. (1993). A yeast mitogen-activated protein kinase homolog (Mpk1p) mediates signalling by protein kinase C. Mol. Cell. Biol. 13, 3067–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K.S., and Levin, D.E. (1992). Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol. Cell. Biol. 12, 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, D.E., and Bartlett-Heubusch, E. (1992). Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J. Cell Biol. 116, 1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, D.E., Fields, F.O., Kunisawa, R., Bishop, J.M., and Thorner, J. (1990). A candidate protein kinase C gene, PKC1, is required for the S. cerevisiae cell cycle. Cell 62, 213–224. [DOI] [PubMed] [Google Scholar]
- Lew, D.J., and Reed, S.I. (1995). A cell cycle checkpoint monitors cell morphogenesis in budding yeast. J. Cell Biol. 129, 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Moir, R.D., Sethy-Coraci, I.K., Warner, J.R., and Willis, I.M. (2000). Repression of ribosome and tRNA synthesis in secretion-defective cells is signaled by a novel branch of the cell integrity pathway. Mol. Cell. Biol. 20, 3842–3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Farmer, J., Jr., Lane, W.S., Friedman, J., Weissman, I., and Schreiber, S.L. (1991). Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66, 807–815. [DOI] [PubMed] [Google Scholar]
- Locke, E.G., Bonilla, M., Liang, L., Takita, Y., and Cunningham, K.W. (2000). A homolog of voltage-gated Ca2+ channels stimulated by depletion of secretory Ca2+ in yeast. Mol. Cell. Biol. 20, 6686–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X.J., Lu, Q., and Grunstein, M. (1996). A search for proteins that interact genetically with histone H3 and H4 amino termini uncovers novel regulators of the Swe1 kinase in Saccharomyces cerevisiae. Genes Dev. 10, 1327–1340. [DOI] [PubMed] [Google Scholar]
- Madden, K., Sheu, Y.J., Baetz, K., Andrews, B., and Snyder, M. (1997). SBF cell cycle regulator as a target of the yeast PKC-MAP kinase pathway. Science 275, 1781–1784. [DOI] [PubMed] [Google Scholar]
- Madeo, F., et al. (2002). A caspase-related protease regulates apoptosis in yeast. Mol. Cell 9, 911–917. [DOI] [PubMed] [Google Scholar]
- Marchetti, O., Entenza, J.M., Sanglard, D., Bille, J., Glauser, M.P., and Moreillon, P. (2000a). Fluconazole plus cyclosporine: a fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob. Agents Chemother. 44, 2932–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti, O., Moreillon, P., Glauser, M.P., Bille, J., and Sanglard, D. (2000b). Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob. Agents. Chemother. 44, 2373–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheos, D.P., Kingsbury, T.J., Ahsan, U.S., and Cunningham, K.W. (1997). Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 11, 3445–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan, J.N., Sia, R.A., Bardes, E.S., and Lew, D.J. (1999). Phosphorylation-independent inhibition of Cdc28p by the tyrosine kinase Swe1p in the morphogenesis checkpoint. Mol. Cell. Biol. 19, 5981–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan, J.N., Sia, R.A., and Lew, D.J. (1998). A morphogenesis checkpoint monitors the actin cytoskeleton in yeast. J. Cell Biol. 142, 1487–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizunuma, M., Hirata, D., Miyahara, K., Tsuchiya, E., and Miyakawa, T. (1998). Role of calcineurin and Mpk1 in regulating the onset of mitosis in budding yeast. Nature 392, 303–306. [DOI] [PubMed] [Google Scholar]
- Mizunuma, M., Hirata, D., Miyaoka, R., and Miyakawa, T. (2001). GSK-3 kinase Mck1 and calcineurin coordinately mediate Hsl1 down-regulation by Ca2+ in budding yeast. EMBO J. 20, 1074–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondesert, G., and Reed, S.I. (1996). BED1, a gene encoding a galactosyltransferase homologue, is required for polarized growth and efficient bud emergence in Saccharomyces cerevisiae. J. Cell Biol. 132, 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, E.M., Locke, E.G., and Cunningham, K.W. (2001). Differential regulation of two Ca2+ influx systems by pheromone signaling in Saccharomyces cerevisiae. Genetics 159, 1527–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri, J., and Tartakoff, A.M. (2001). The arrest of secretion response in yeast: signaling from the secretory path to the nucleus via Wsc proteins and Pkc1p. Mol. Cell 8, 281–289. [DOI] [PubMed] [Google Scholar]
- Nierras, C.R., and Warner, J.R. (1999). Protein kinase C enables the regulatory circuit that connects membrane synthesis to ribosome synthesis in Saccharomyces cerevisiae. J. Biol. Chem. 274, 13235–13241. [DOI] [PubMed] [Google Scholar]
- Nonaka, H., Tanaka, K., Hirano, H., Fujiwara, T., Kohno, H., Umikawa, M., Mino, A., and Takai, Y. (1995). A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 14, 5931–5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono, T., Suzuki, T., Anraku, Y., and Iida, H. (1994). The MID2 gene encodes a putative integral membrane protein with a Ca2+-binding domain and shows mating pheromone-stimulated expression in Saccharomyces cerevisiae. Gene 151, 203–208. [DOI] [PubMed] [Google Scholar]
- Paidhungat, M., and Garrett, S. (1997). A homolog of mammalian, voltage-gated calcium channels mediates yeast pheromone-stimulated Ca2+ uptake and exacerbates the cdc1(Ts) growth defect. Mol. Cell. Biol. 17, 6339–6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil, C., and Walter, P. (2001). Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr. Opin. Cell Biol. 13, 349–355. [DOI] [PubMed] [Google Scholar]
- Philip, B., and Levin, D.E. (2001). Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol. Cell. Biol. 21, 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajavel, M., Philip, B., Buehrer, B.M., Errede, B., and Levin, D.E. (1999). Mid2 is a putative sensor for cell integrity signaling in Saccharomyces cerevisiae. Mol. Cell. Biol. 19, 3969–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, P., Moreno, S., and Reed, S.I. (1989). Conservation of mitotic controls in fission and budding yeasts. Cell 57, 295–303. [DOI] [PubMed] [Google Scholar]
- Sherman, F., Hicks, J.B., and Fink, G.R. (1986). Methods in Yeast Genetics, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
- Sidrauski, C., Chapman, R., and Walter, P. (1998). The unfolded protein response: an intracellular signalling pathway with many surprising features. Trends Cell Biol. 8, 245–249. [DOI] [PubMed] [Google Scholar]
- Stathopoulos, A.M., and Cyert, M.S. (1997). Calcineurin acts through the CRZ1/TCN1 encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11, 3432–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterlin, C., Hsu, P., Mallabiabarrena, A., and Malhotra, V. (2002). Fragmentation and dispersal of the pericentriolar Golgi complex is required for entry into mitosis in mammalian cells. Cell 109, 359–369. [DOI] [PubMed] [Google Scholar]
- Tanaka, S., and Nojima, H. (1996). Nik 1, a Nim1-like protein kinase of S. cerevisiae interacts with the Cdc28 complex and regulates cell cycle progression. Genes Cells 1, 905–921. [DOI] [PubMed] [Google Scholar]
- Torres, J., Di Como, C.J., Herrero, E., and de la Torre-Ruiz, M.A. (2002). Regulation of the cell integrity pathway by rapamycin-sensitive TOR function in budding yeast. J. Biol. Chem. 277, 43495–43504. [DOI] [PubMed] [Google Scholar]
- Torres, L., Martin, H., Garcia-Saez, M.I., Arroyo, J., Molina, M., Sanchez, M., and Nombela, C. (1991). A protein kinase gene complements the lytic phenotype of Saccharomyces cerevisiae lyt2 mutants. Mol. Microbiol. 5, 2845–2854. [DOI] [PubMed] [Google Scholar]
- Travers, K.J., Patil, C.K., Wodicka, L., Lockhart, D.J., Weissman, J.S., and Walter, P. (2000). Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101, 249–258. [DOI] [PubMed] [Google Scholar]
- Vai, M., Popolo, L., and Alberghina, L. (1987). Effect of tunicamycin on cell cycle progression in budding yeast. Exp. Cell Res. 171, 448–459. [DOI] [PubMed] [Google Scholar]
- Verna, J., Lodder, A., Lee, K., Vagts, A., and Ballester, R. (1997). A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 94, 13804–13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis, J.W., Chrebet, G., Brodsky, G., Rolfe, M., and Rothstein, R. (1989). A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell 58, 409–419. [DOI] [PubMed] [Google Scholar]
- Watanabe, M., Chen, C.Y., and Levin, D.E. (1994). Saccharomyces cerevisiae PKC1 encodes a protein kinase C (PKC) homolog with a substrate specificity similar to that of mammalian PKC. J. Biol. Chem. 269, 16829–16836. [PubMed] [Google Scholar]
- Watanabe, Y., Takaesu, G., Hagiwara, M., Irie, K., and Matsumoto, K. (1997). Characterization of a serum response factor-like protein in Saccharomyces cerevisiae, Rlm1, which has transcriptional activity regulated by the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 17, 2615–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakel, J.L. (1997). Calcineurin regulation of synaptic function: from ion channels to transmitter release and gene transcription. Trends Pharmacol. Sci. 18, 124–134. [DOI] [PubMed] [Google Scholar]
- Yoshimoto, H., Saltsman, K., Gasch, A.P., Li, H.X., Ogawa, N., Botstein, D., Brown, P.O., and Cyert, M.S. (2002). Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J. Biol. Chem. 277, 31079–31088. [DOI] [PubMed] [Google Scholar]
- Zhao, C., Jung, U.S., Garrett-Engele, P., Roe, T., Cyert, M.S., and Levin, D.E. (1998). Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol. Cell. Biol. 18, 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]