Abstract
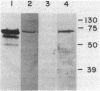
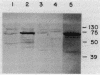
Choline oxidase (EC 1.1.3.17) is a bifunctional enzyme that is capable of catalyzing glycine betaine biosynthesis from choline via betaine aldehyde. A gene (cox) encoding this enzyme in the gram-positive soil bacterium Arthrobacter pascens was isolated and characterized. This gene is contained within a 1.9-kb fragment that encodes a polypeptide of approximately 66 kDa. Transfer of this gene to an Escherichia coli mutant that is defective in betaine biosynthesis resulted in an osmotolerant phenotype. This phenotype was associated with the ability of the host to synthesize and assemble an enzymatically active choline oxidase that could catalyze biosynthesis of glycine betaine from an exogenous supply of choline. Although glycine betaine functions as an osmolyte in several different organisms, it was not found to have this role in A. pascens. Instead, both choline and glycine betaine were utilized as carbon sources. In A. pascens synthesis and activity of choline oxidase were modulated by carbon sources and were susceptible to catabolite repression. Thus, cox, a gene concerned with carbon utilization in A. pascens, was found to play a role in adaptation to an environmental stress in a heterologous organism. In addition to providing a possible means of manipulating osmotolerance in other organisms, the cox gene offers a model system for the study of choline oxidation, an important metabolic process in both procaryotes and eucaryotes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andresen P. A., Kaasen I., Styrvold O. B., Boulnois G., Strøm A. R. Molecular cloning, physical mapping and expression of the bet genes governing the osmoregulatory choline-glycine betaine pathway of Escherichia coli. J Gen Microbiol. 1988 Jun;134(6):1737–1746. doi: 10.1099/00221287-134-6-1737. [DOI] [PubMed] [Google Scholar]
- Bagnasco S., Balaban R., Fales H. M., Yang Y. M., Burg M. Predominant osmotically active organic solutes in rat and rabbit renal medullas. J Biol Chem. 1986 May 5;261(13):5872–5877. [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brosius J., Holy A. Regulation of ribosomal RNA promoters with a synthetic lac operator. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6929–6933. doi: 10.1073/pnas.81.22.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouquisse R., Weigel P., Rhodes D., Yocum C. F., Hanson A. D. Evidence for a ferredoxin-dependent choline monooxygenase from spinach chloroplast stroma. Plant Physiol. 1989 May;90(1):322–329. doi: 10.1104/pp.90.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Byerrum R. U., Sato C. S., Ball C. D. Utilization of Betaine as a Methyl Group Donor in Tobacco. Plant Physiol. 1956 Sep;31(5):374–377. doi: 10.1104/pp.31.5.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella L., Tomassetti M., De Angelis G., Sammartino M. P., Cordatore M. A new assay for choline-containing phospholipids in amniotic fluid by an enzyme sensor. Clin Chim Acta. 1987 Nov 16;169(2-3):175–182. doi: 10.1016/0009-8981(87)90317-2. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G., Rapatz W., Ruis H. Sequence of the Saccharomyces cerevisiae CTA1 gene and amino acid sequence of catalase A derived from it. Eur J Biochem. 1988 Sep 1;176(1):159–163. doi: 10.1111/j.1432-1033.1988.tb14263.x. [DOI] [PubMed] [Google Scholar]
- Cohen G., Rapatz W., Ruis H. Sequence of the Saccharomyces cerevisiae CTA1 gene and amino acid sequence of catalase A derived from it. Eur J Biochem. 1988 Sep 1;176(1):159–163. doi: 10.1111/j.1432-1033.1988.tb14263.x. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- Haubrich D. R., Gerber N. H. Choline dehydrogenase. Assay, properties and inhibitors. Biochem Pharmacol. 1981 Nov 1;30(21):2993–3000. doi: 10.1016/0006-2952(81)90265-3. [DOI] [PubMed] [Google Scholar]
- Ikuta S., Imamura S., Misaki H., Horiuti Y. Purification and characterization of choline oxidase from Arthrobacter globiformis. J Biochem. 1977 Dec;82(6):1741–1749. doi: 10.1093/oxfordjournals.jbchem.a131872. [DOI] [PubMed] [Google Scholar]
- Ikuta S., Matuura K., Imamura S., Misaki H., Horiuti Y. Oxidative pathway of choline to betaine in the soluble fraction prepared from Arthrobacter globiformis. J Biochem. 1977 Jul;82(1):157–163. doi: 10.1093/oxfordjournals.jbchem.a131664. [DOI] [PubMed] [Google Scholar]
- Landfald B., Strøm A. R. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J Bacteriol. 1986 Mar;165(3):849–855. doi: 10.1128/jb.165.3.849-855.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Morrison D. A. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 1979;68:326–331. doi: 10.1016/0076-6879(79)68023-0. [DOI] [PubMed] [Google Scholar]
- Ohta-Fukuyama M., Miyake Y., Emi S., Yamano T. Identification and properties of the prosthetic group of choline oxidase from Alcaligenes sp. J Biochem. 1980 Jul;88(1):197–203. [PubMed] [Google Scholar]
- Perroud B., Le Rudulier D. Glycine betaine transport in Escherichia coli: osmotic modulation. J Bacteriol. 1985 Jan;161(1):393–401. doi: 10.1128/jb.161.1.393-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHSCHILD H. A., BARRON E. S. G. The oxidation of betaine aldehyde by betaine aldehyde dehydrogenase. J Biol Chem. 1954 Aug;209(2):511–523. [PubMed] [Google Scholar]
- Repaske R., Repaske A. C. Quantitative requirements for exponential growth of Alcaligenes eutrophus. Appl Environ Microbiol. 1976 Oct;32(4):585–591. doi: 10.1128/aem.32.4.585-591.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E., Kolattukudy P. E. Molecular cloning, nucleotide sequence, and abscisic acid induction of a suberization-associated highly anionic peroxidase. Mol Gen Genet. 1989 Jun;217(2-3):223–232. doi: 10.1007/BF02464885. [DOI] [PubMed] [Google Scholar]
- Robertson D. E., Noll D., Roberts M. F., Menaia J. A., Boone D. R. Detection of the osmoregulator betaine in methanogens. Appl Environ Microbiol. 1990 Feb;56(2):563–565. doi: 10.1128/aem.56.2.563-565.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiba W. E., Taylor M. P., Wells M. S., Mangum J. H., Awad W. M., Jr Human hepatic methionine biosynthesis. Purification and characterization of betaine:homocysteine S-methyltransferase. J Biol Chem. 1982 Dec 25;257(24):14944–14948. [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. T., Pocard J. A., Bernard T., Le Rudulier D. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J Bacteriol. 1988 Jul;170(7):3142–3149. doi: 10.1128/jb.170.7.3142-3149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. S., Lubinsky-Mink S., Jackson C. G., Cassel A., Kuhn J. pHG165: a pBR322 copy number derivative of pUC8 for cloning and expression. Plasmid. 1986 May;15(3):172–181. doi: 10.1016/0147-619x(86)90035-1. [DOI] [PubMed] [Google Scholar]
- Styrvold O. B., Falkenberg P., Landfald B., Eshoo M. W., Bjørnsen T., Strøm A. R. Selection, mapping, and characterization of osmoregulatory mutants of Escherichia coli blocked in the choline-glycine betaine pathway. J Bacteriol. 1986 Mar;165(3):856–863. doi: 10.1128/jb.165.3.856-863.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama M., Itoh S., Nagasaki T., Tanimizu I. A new enzymatic method for determination of serum choline-containing phospholipids. Clin Chim Acta. 1977 Aug 15;79(1):93–98. doi: 10.1016/0009-8981(77)90465-x. [DOI] [PubMed] [Google Scholar]
- Triggs-Raine B. L., Doble B. W., Mulvey M. R., Sorby P. A., Loewen P. C. Nucleotide sequence of katG, encoding catalase HPI of Escherichia coli. J Bacteriol. 1988 Sep;170(9):4415–4419. doi: 10.1128/jb.170.9.4415-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. F., Demain A. L. Catabolism of betaine and its relationship to cobalamin overproduction. Biochim Biophys Acta. 1971 Apr 20;237(1):112–119. doi: 10.1016/0304-4165(71)90036-5. [DOI] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]