Abstract
A stable extrachromosomally maintained transformation vector (pG12-1) for the lignin-degrading filamentous fungus Phanerochaete chrysosporium is described. The vector is 6.3 kb and contains a Kanr marker, pBR322 ori, and a 2.2-kb fragment (ME-1) derived from an endogenous extrachromosomal DNA element of P. chrysosporium. Vector pG12-1 was able to transform P. chrysosporium to G418 resistance and was readily and consistently recoverable from the total DNA of transformants via Escherichia coli transformation. Southern blot analyses indicated that pG12-1 is maintained at a low copy number in the fungal transformants. The vector is demonstrable in the total DNA of individual G418-resistant basidiospore progeny of the transformants only after amplification by polymerase chain reaction. Exonuclease III and dam methylation analyses, respectively, indicated that pG12-I undergoes replication in P. chrysosporium and that it is maintained extrachromosomally in a circular form. The vector is stably maintained in the transformants even after long-term nonselective growth. There is no evidence for integration of the vector into the chromosome at any stage.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alic M., Kornegay J. R., Pribnow D., Gold M. H. Transformation by Complementation of an Adenine Auxotroph of the Lignin-Degrading Basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1989 Feb;55(2):406–411. doi: 10.1128/aem.55.2.406-411.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrawis A., Pease E. A., Kuan I. C., Holzbaur E., Tien M. Characterization of two lignin peroxidase clones from Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1989 Jul 31;162(2):673–680. doi: 10.1016/0006-291x(89)92363-2. [DOI] [PubMed] [Google Scholar]
- Arnau J., Murillo F. J., Torres-Martínez S. Expression of Tn5-derived kanamycin resistance in the fungus Phycomyces blakesleeanus. Mol Gen Genet. 1988 May;212(2):375–377. doi: 10.1007/BF00334710. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boominathan K., Dass S. B., Randall T. A., Kelley R. L., Reddy C. A. Lignin peroxidase-negative mutant of the white-rot basidiomycete Phanerochaete chrysosporium. J Bacteriol. 1990 Jan;172(1):260–265. doi: 10.1128/jb.172.1.260-265.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A., Sims P. F., Raeder U., Broda P. Multiple ligninase-related genes from Phanerochaete chrysosporium. Gene. 1988 Dec 15;73(1):77–85. doi: 10.1016/0378-1119(88)90314-9. [DOI] [PubMed] [Google Scholar]
- Bull J. H., Wootton J. C. Heavily methylated amplified DNA in transformants of Neurospora crassa. Nature. 1984 Aug 23;310(5979):701–704. doi: 10.1038/310701a0. [DOI] [PubMed] [Google Scholar]
- Bumpus J. A., Aust S. D. Biodegradation of DDT [1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane] by the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1987 Sep;53(9):2001–2008. doi: 10.1128/aem.53.9.2001-2008.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus J. A., Tien M., Wright D., Aust S. D. Oxidation of persistent environmental pollutants by a white rot fungus. Science. 1985 Jun 21;228(4706):1434–1436. doi: 10.1126/science.3925550. [DOI] [PubMed] [Google Scholar]
- Claverys J. P., Lacks S. A. Heteroduplex deoxyribonucleic acid base mismatch repair in bacteria. Microbiol Rev. 1986 Jun;50(2):133–165. doi: 10.1128/mr.50.2.133-165.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. H., Cheng T. M., Alic M. Formation, Fusion, and Regeneration of Protoplasts from Wild-Type and Auxotrophic Strains of the White Rot Basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1983 Jul;46(1):260–263. doi: 10.1128/aem.46.1.260-263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. H., Cheng T. M., Mayfield M. B. Isolation and Complementation Studies of Auxotrophic Mutants of the Lignin-Degrading Basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1982 Oct;44(4):996–1000. doi: 10.1128/aem.44.4.996-1000.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley N. D., Joyce C. M. Genetic and DNA sequence analysis of the kanamycin resistance transposon Tn903. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7176–7180. doi: 10.1073/pnas.77.12.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Holst A., Müller F., Zastrow G., Zentgraf H., Schwender S., Dinkl E., Grummt F. Murine genomic DNA sequences replicating autonomously in mouse L cells. Cell. 1988 Feb 12;52(3):355–365. doi: 10.1016/s0092-8674(88)80028-x. [DOI] [PubMed] [Google Scholar]
- Holzbaur E. L., Andrawis A., Tien M. Structure and regulation of a lignin peroxidase gene from Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1988 Sep 15;155(2):626–633. doi: 10.1016/s0006-291x(88)80541-2. [DOI] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Liwicki R., Paterson A., MacDonald M. J., Broda P. Phenotypic classes of phenoloxidase-negative mutants of the lignin-degrading fungus Phanerochaete chrysosporium. J Bacteriol. 1985 May;162(2):641–644. doi: 10.1128/jb.162.2.641-644.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molskness T. A., Alic M., Gold M. H. Characterization of Leucine Auxotrophs of the White Rot Basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1986 Jun;51(6):1170–1173. doi: 10.1128/aem.51.6.1170-1173.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Szostak J. W. Pedigree analysis of plasmid segregation in yeast. Cell. 1983 Oct;34(3):961–970. doi: 10.1016/0092-8674(83)90553-6. [DOI] [PubMed] [Google Scholar]
- Oka A., Sugisaki H., Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981 Apr 5;147(2):217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- Paietta J., Marzluf G. A. Plasmid recovery from transformants and the isolation of chromosomal DNA segments improving plasmid replication in Neurospora crassa. Curr Genet. 1985;9(5):383–388. doi: 10.1007/BF00421609. [DOI] [PubMed] [Google Scholar]
- Pease E. A., Andrawis A., Tien M. Manganese-dependent peroxidase from Phanerochaete chrysosporium. Primary structure deduced from cDNA sequence. J Biol Chem. 1989 Aug 15;264(23):13531–13535. [PubMed] [Google Scholar]
- Perrot M., Barreau C., Bégueret J. Nonintegrative transformation in the filamentous fungus Podospora anserina: stabilization of a linear vector by the chromosomal ends of Tetrahymena thermophila. Mol Cell Biol. 1987 May;7(5):1725–1730. doi: 10.1128/mcb.7.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W. A., Kistler H. C. In vivo rearrangement of foreign DNA by Fusarium oxysporum produces linear self-replicating plasmids. J Bacteriol. 1990 Jun;172(6):3163–3171. doi: 10.1128/jb.172.6.3163-3171.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribnow D., Mayfield M. B., Nipper V. J., Brown J. A., Gold M. H. Characterization of a cDNA encoding a manganese peroxidase, from the lignin-degrading basidiomycete Phanerochaete chrysosporium. J Biol Chem. 1989 Mar 25;264(9):5036–5040. [PubMed] [Google Scholar]
- Randall T., Rao T. R., Reddy C. A. Use of a shuttle vector for the transformation of the white rot basidiomycete, Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1989 Jun 15;161(2):720–725. doi: 10.1016/0006-291x(89)92659-4. [DOI] [PubMed] [Google Scholar]
- Rao T. R., Reddy C. A. DNA sequences from a ligninolytic filamentous fungus Phanerochaete chrysosporium capable of autonomous replication in yeast. Biochem Biophys Res Commun. 1984 Feb 14;118(3):821–827. doi: 10.1016/0006-291x(84)91468-2. [DOI] [PubMed] [Google Scholar]
- Razanamparany V., Bégueret J. Positive screening and transformation of ura5 mutants in the fungus Podospora anserina: characterization of the transformants. Curr Genet. 1986;10(11):811–817. doi: 10.1007/BF00418527. [DOI] [PubMed] [Google Scholar]
- Revuelta J. L., Jayaram M. Transformation of Phycomyces blakesleeanus to G-418 resistance by an autonomously replicating plasmid. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7344–7347. doi: 10.1073/pnas.83.19.7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncero M. I., Jepsen L. P., Strøman P., van Heeswijck R. Characterization of a leuA gene and an ARS element from Mucor circinelloides. Gene. 1989 Dec 14;84(2):335–343. doi: 10.1016/0378-1119(89)90508-8. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Schalch H., Gaskell J., Smith T. L., Cullen D. Molecular cloning and sequences of lignin peroxidase genes of Phanerochaete chrysosporium. Mol Cell Biol. 1989 Jun;9(6):2743–2747. doi: 10.1128/mcb.9.6.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
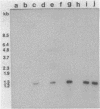
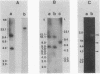
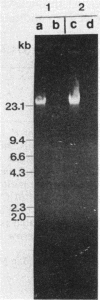
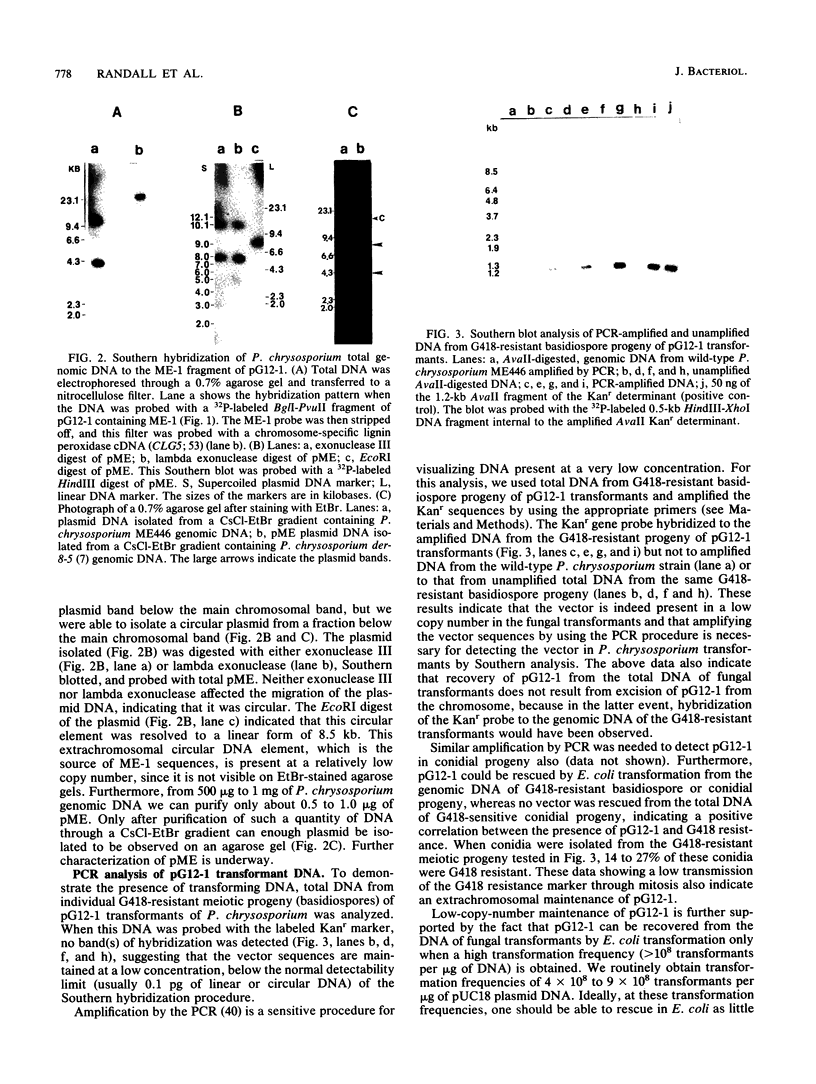
- Smith T. L., Schalch H., Gaskell J., Covert S., Cullen D. Nucleotide sequence of a ligninase gene from Phanerochaete chrysosporium. Nucleic Acids Res. 1988 Feb 11;16(3):1219–1219. doi: 10.1093/nar/16.3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl L. L., Akins R. A., Lambowitz A. M. Characterization of deletion derivatives of an autonomously replicating Neurospora plasmid. Nucleic Acids Res. 1984 Aug 10;12(15):6169–6178. doi: 10.1093/nar/12.15.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl L. L., Lambowitz A. M. Construction of a shuttle vector for the filamentous fungus Neurospora crassa. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1058–1062. doi: 10.1073/pnas.80.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M. Properties of ligninase from Phanerochaete chrysosporium and their possible applications. Crit Rev Microbiol. 1987;15(2):141–168. doi: 10.3109/10408418709104456. [DOI] [PubMed] [Google Scholar]
- Tien M., Tu C. P. Cloning and sequencing of a cDNA for a ligninase from Phanerochaete chrysosporium. Nature. 1987 Apr 2;326(6112):520–523. doi: 10.1038/326520a0. [DOI] [PubMed] [Google Scholar]
- Tsukuda T., Carleton S., Fotheringham S., Holloman W. K. Isolation and characterization of an autonomously replicating sequence from Ustilago maydis. Mol Cell Biol. 1988 Sep;8(9):3703–3709. doi: 10.1128/mcb.8.9.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther I., Kälin M., Reiser J., Suter F., Fritsche B., Saloheimo M., Leisola M., Teeri T., Knowles J. K., Fiechter A. Molecular analysis of a Phanerochaete chrysosporium lignin peroxidase gene. Gene. 1988 Oct 15;70(1):127–137. doi: 10.1016/0378-1119(88)90111-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Z., Zylstra G. J., Olsen R. H., Reddy C. A. Identification of cDNA clones for ligninase from Phanerochaete chrysosporium using synthetic oligonucleotide probes. Biochem Biophys Res Commun. 1986 Jun 13;137(2):649–656. doi: 10.1016/0006-291x(86)91127-7. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Zhang Y. Z., Collins C., Reddy C. A. Analysis of nucleotide sequences of two ligninase cDNAs from a white-rot filamentous fungus, Phanerochaete chrysosporium. Gene. 1987;60(1):93–102. doi: 10.1016/0378-1119(87)90217-4. [DOI] [PubMed] [Google Scholar]