Abstract
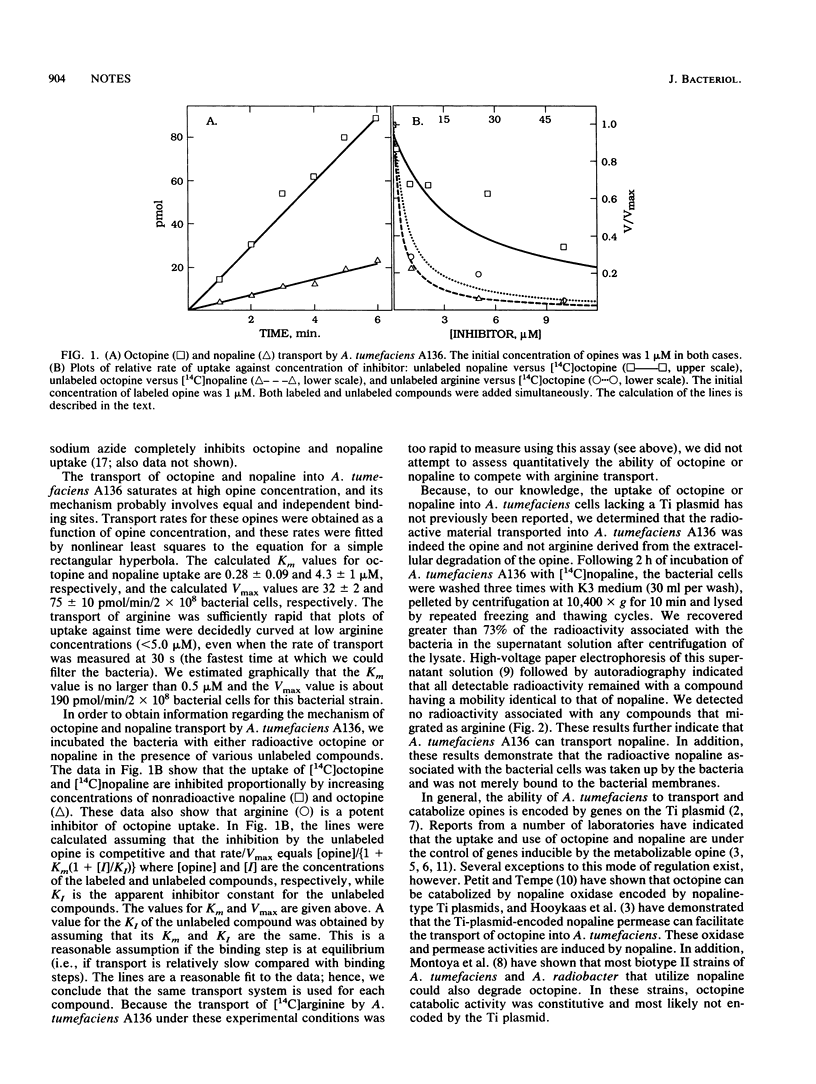
We have examined the uptake of [14C]octopine and [14C]nopaline by Agrobacterium tumefaciens strains containing the C58 chromosomal background in medium suitable for the induction of vir genes. All strains tested could transport both of these opines, regardless of the presence or type of Ti plasmid (octopine or nopaline) present in the bacterium. The transport of these opines required active cellular metabolism. Nonradioactive octopine, nopaline, and arginine competed effectively with [14C]octopine and [14C]nopaline for transport into A. tumefaciens A136, suggesting that the transport of these opines occurs via an arginine transport pathway not encoded by the Ti plasmid.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton G. W., Nester E. W., Gordon M. P. Plant phenolic compounds induce expression of the Agrobacterium tumefaciens loci needed for virulence. Science. 1986 May 23;232(4753):983–985. doi: 10.1126/science.3085219. [DOI] [PubMed] [Google Scholar]
- Bomhoff G., Klapwijk P. M., Kester H. C., Schilperoort R. A., Hernalsteens J. P., Schell J. Octopine and nopaline synthesis and breakdown genetically controlled by a plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1976 May 7;145(2):177–181. doi: 10.1007/BF00269591. [DOI] [PubMed] [Google Scholar]
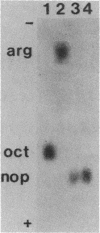
- Jensen R. E., Zdybak W. T., Yasuda K., Chilton W. S. A useful synthesis of nopaline, a crown gall tumor metabolite. Biochem Biophys Res Commun. 1977 Apr 25;75(4):1066–1070. doi: 10.1016/0006-291x(77)91490-5. [DOI] [PubMed] [Google Scholar]
- Klapwijk P. M., Schilperoort R. A. Negative control of octopine degradation and transfer genes of octopine Ti plasmids in Agrobacterium tumefaciens. J Bacteriol. 1979 Aug;139(2):424–431. doi: 10.1128/jb.139.2.424-431.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya A. L., Chilton M. D., Gordon M. P., Sciaky D., Nester E. W. Octopine and nopaline metabolism in Agrobacterium tumefaciens and crown gall tumor cells: role of plasmid genes. J Bacteriol. 1977 Jan;129(1):101–107. doi: 10.1128/jb.129.1.101-107.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya A. L., Moore L. W., Gordon M. P., Nester E. W. Multiple genes coding for octopine-degrading enzymes in Agrobacterium. J Bacteriol. 1978 Dec;136(3):909–915. doi: 10.1128/jb.136.3.909-915.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten L. A., Schilperoort R. A. A rapid micro scale method for the detection of lysopine and nopaline dehydrogenase activities. Biochim Biophys Acta. 1978 Dec 8;527(2):497–500. doi: 10.1016/0005-2744(78)90363-7. [DOI] [PubMed] [Google Scholar]
- Stachel S. E., Zambryski P. C. Agrobacterium tumefaciens and the susceptible plant cell: a novel adaptation of extracellular recognition and DNA conjugation. Cell. 1986 Oct 24;47(2):155–157. doi: 10.1016/0092-8674(86)90437-x. [DOI] [PubMed] [Google Scholar]
- Veluthambi K., Krishnan M., Gould J. H., Smith R. H., Gelvin S. B. Opines stimulate induction of the vir genes of the Agrobacterium tumefaciens Ti plasmid. J Bacteriol. 1989 Jul;171(7):3696–3703. doi: 10.1128/jb.171.7.3696-3703.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veluthambi K., Ream W., Gelvin S. B. Virulence genes, borders, and overdrive generate single-stranded T-DNA molecules from the A6 Ti plasmid of Agrobacterium tumefaciens. J Bacteriol. 1988 Apr;170(4):1523–1532. doi: 10.1128/jb.170.4.1523-1532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]