Abstract
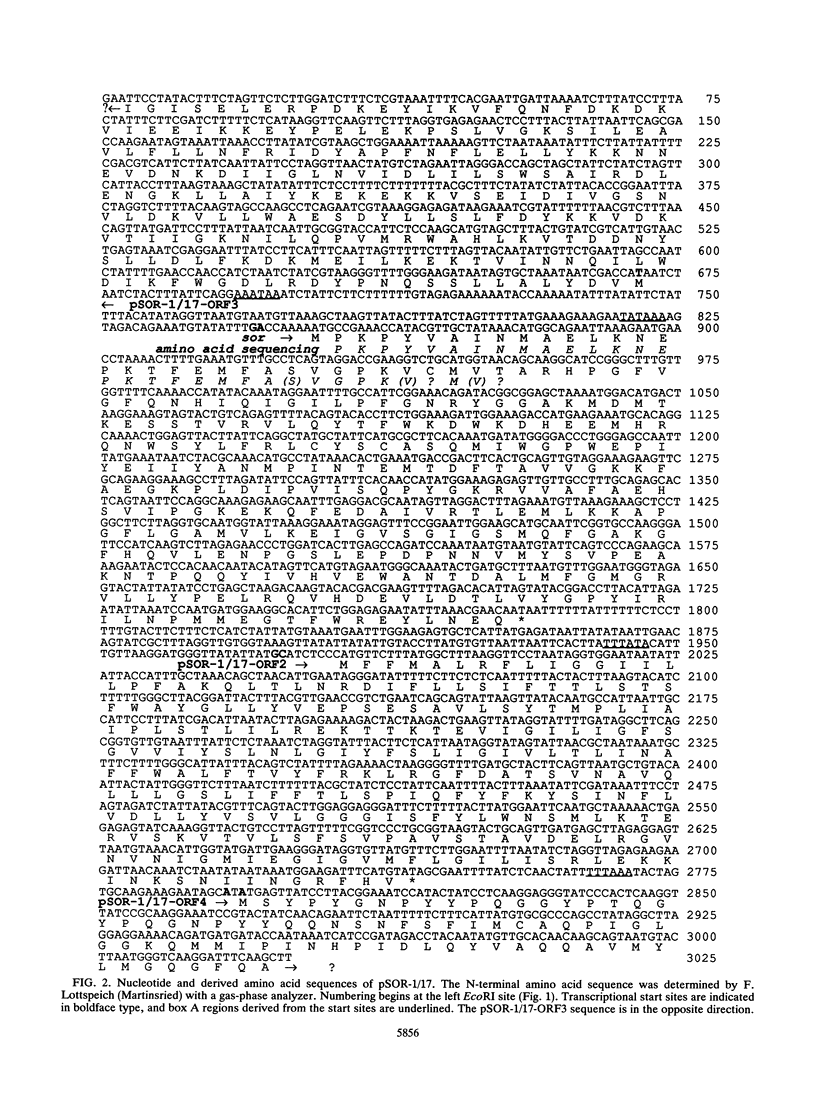
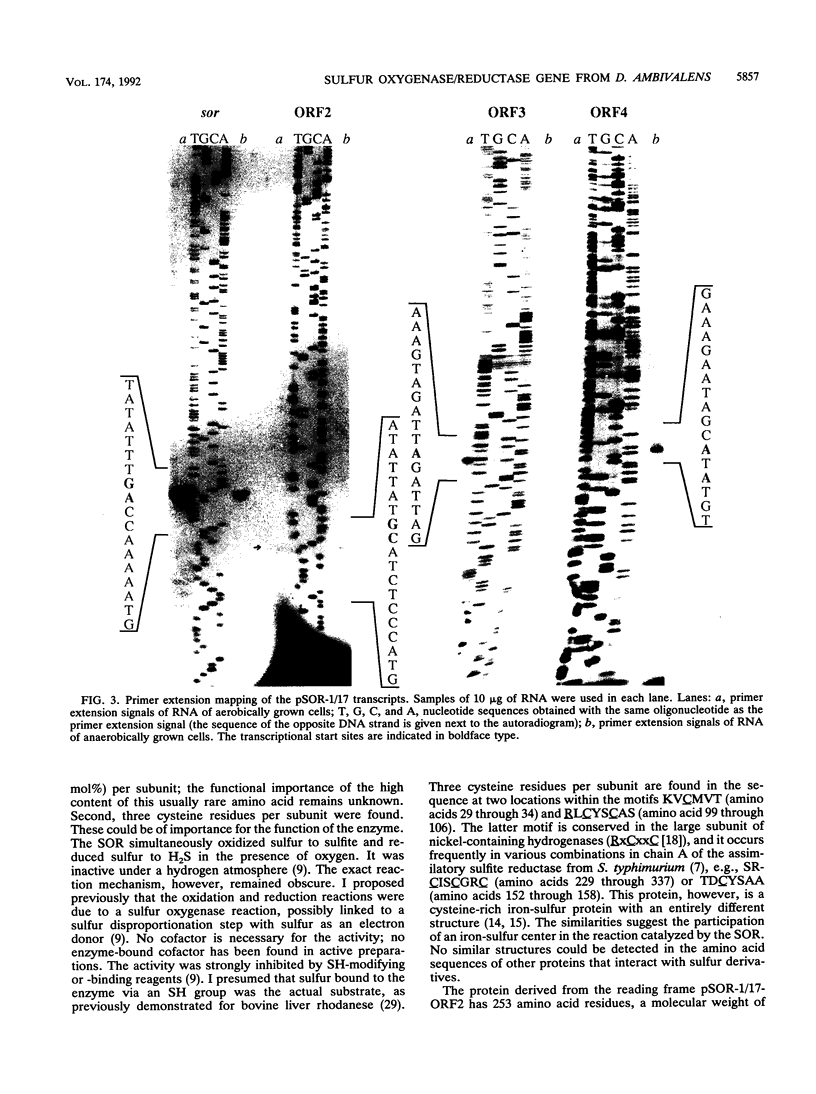
A 5.8-kbp HindIII fragment containing the sor gene which encodes the aerobically induced sulfur oxygenase/reductase of the thermoacidophilic, chemolithoautotrophic, and facultatively anaerobic archaeum Desulfurolobus ambivalens, was cloned in pUC18 by using an oligonucleotide derived from the N-terminal amino acid sequence for identification (pSOR-1/17). The native enzyme is a 550,000-molecular-weight oligomer composed of single 40,000-molecular-weight subunits; this oligomer is capable of the simultaneous oxidation and reduction of sulfur (A. Kletzin, J. Bacteriol. 171:1638-1643, 1989). From the fragment, 3,025 bp that contained the entire sor gene were sequenced. The sor gene encoded a protein with 309 amino acid residues (molecular weight, 35,317). The transcript length was determined by Northern RNA hybridization to be 960 to 1,020 nucleotides, and the transcriptional start site was mapped by primer extension analysis. The transcript of the sor gene in aerobically grown cells was amplified 38- to 42-fold relative to that in anaerobically grown cells. An initial transcriptional characterization of three neighboring genes of unknown function is also reported.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer F., Zillig W., Stetter K. O., Schreiber G. Chemolithoautotrophic metabolism of anaerobic extremely thermophilic archaebacteria. Nature. 1983 Feb 10;301(5900):511–513. doi: 10.1038/301511a0. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Huang C. J., Barrett E. L. Sequence analysis and expression of the Salmonella typhimurium asr operon encoding production of hydrogen sulfide from sulfite. J Bacteriol. 1991 Feb;173(4):1544–1553. doi: 10.1128/jb.173.4.1544-1553.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüdepohl U., Reiter W. D., Zillig W. In vitro transcription of two rRNA genes of the archaebacterium Sulfolobus sp. B12 indicates a factor requirement for specific initiation. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5851–5855. doi: 10.1073/pnas.87.15.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzin A. Coupled enzymatic production of sulfite, thiosulfate, and hydrogen sulfide from sulfur: purification and properties of a sulfur oxygenase reductase from the facultatively anaerobic archaebacterium Desulfurolobus ambivalens. J Bacteriol. 1989 Mar;171(3):1638–1643. doi: 10.1128/jb.171.3.1638-1643.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafft T., Bokranz M., Klimmek O., Schröder I., Fahrenholz F., Kojro E., Kröger A. Cloning and nucleotide sequence of the psrA gene of Wolinella succinogenes polysulphide reductase. Eur J Biochem. 1992 Jun 1;206(2):503–510. doi: 10.1111/j.1432-1033.1992.tb16953.x. [DOI] [PubMed] [Google Scholar]
- Krone F. A., Westphal G., Schwenn J. D. Characterisation of the gene cysH and of its product phospho-adenylylsulphate reductase from Escherichia coli. Mol Gen Genet. 1991 Feb;225(2):314–319. doi: 10.1007/BF00269864. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Ostrowski J., Wu J. Y., Rueger D. C., Miller B. E., Siegel L. M., Kredich N. M. Characterization of the cysJIH regions of Salmonella typhimurium and Escherichia coli B. DNA sequences of cysI and cysH and a model for the siroheme-Fe4S4 active center of sulfite reductase hemoprotein based on amino acid homology with spinach nitrite reductase. J Biol Chem. 1989 Sep 15;264(26):15726–15737. [PubMed] [Google Scholar]
- Ostrowski J., Wu J. Y., Rueger D. C., Miller B. E., Siegel L. M., Kredich N. M. Characterization of the cysJIH regions of Salmonella typhimurium and Escherichia coli B. DNA sequences of cysI and cysH and a model for the siroheme-Fe4S4 active center of sulfite reductase hemoprotein based on amino acid homology with spinach nitrite reductase. J Biol Chem. 1989 Sep 15;264(26):15726–15737. [PubMed] [Google Scholar]
- Pearson W. R. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 1990;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- Przybyla A. E., Robbins J., Menon N., Peck H. D., Jr Structure-function relationships among the nickel-containing hydrogenases. FEMS Microbiol Rev. 1992 Feb;8(2):109–135. doi: 10.1111/j.1574-6968.1992.tb04960.x. [DOI] [PubMed] [Google Scholar]
- Reiter W. D., Palm P., Zillig W. Analysis of transcription in the archaebacterium Sulfolobus indicates that archaebacterial promoters are homologous to eukaryotic pol II promoters. Nucleic Acids Res. 1988 Jan 11;16(1):1–19. doi: 10.1093/nar/16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter W. D., Palm P., Zillig W. Transcription termination in the archaebacterium Sulfolobus: signal structures and linkage to transcription initiation. Nucleic Acids Res. 1988 Mar 25;16(6):2445–2459. doi: 10.1093/nar/16.6.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J., Weng L., Keim P. S., Heinrikson R. L. The covalent structure of bovine liver rhodanese. Isolation and partial structural analysis of cyanogen bromide fragements and the complete sequence of the enzyme. J Biol Chem. 1978 Nov 25;253(22):8102–8108. [PubMed] [Google Scholar]
- Segerer A., Stetter K. O., Klink F. Two contrary modes of chemolithotrophy in the same archaebacterium. 1985 Feb 28-Mar 6Nature. 313(6005):787–789. doi: 10.1038/313787a0. [DOI] [PubMed] [Google Scholar]
- Shivvers D. W., Brock T. D. Oxidation of elemental sulfur by Sulfolobus acidocaldarius. J Bacteriol. 1973 May;114(2):706–710. doi: 10.1128/jb.114.2.706-710.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J., Helms L. R., Swenson R. P., Cowan J. A. Primary structure of the assimilatory-type sulfite reductase from Desulfovibrio vulgaris (Hildenborough): cloning and nucleotide sequence of the reductase gene. Biochemistry. 1991 Oct 15;30(41):9900–9907. doi: 10.1021/bi00105a013. [DOI] [PubMed] [Google Scholar]
- Weng L., Heinrikson R. L., Westley J. Active site cysteinyl and arginyl residues of rhodanese. A novel formation of disulfide bonds in the active site promoted by phenylglyoxal. J Biol Chem. 1978 Nov 25;253(22):8109–8119. [PubMed] [Google Scholar]
- Wich G., Hummel H., Jarsch M., Bär U., Böck A. Transcription signals for stable RNA genes in Methanococcus. Nucleic Acids Res. 1986 Mar 25;14(6):2459–2479. doi: 10.1093/nar/14.6.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillig W., Yeats S., Holz I., Böck A., Gropp F., Rettenberger M., Lutz S. Plasmid-related anaerobic autotrophy of the novel archaebacterium Sulfolobus ambivalens. 1985 Feb 28-Mar 6Nature. 313(6005):789–791. doi: 10.1038/313789a0. [DOI] [PubMed] [Google Scholar]



