Abstract
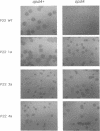
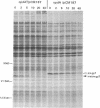
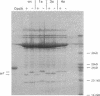
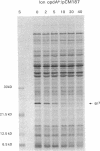
The opdA gene of Salmonella typhimurium encodes an endoprotease, oligopeptidase A (OpdA). Strains carrying opdA mutations were deficient as hosts for phage P22. P22 and the closely related phages L and A3 formed tiny plaques on an opdA host. Salmonella phages 9NA, KB1, and ES18.h1 were not affected by opdA mutations. Although opdA strains displayed normal doubling times and were infected by P22 as efficiently as opdA+ strains, the burst size of infectious particles from an opdA host was less than 1/10 of that from an opdA+ host. This decrease resulted from a reduced efficiency of plating of particles from an opdA infection. In the absence of a functional opdA gene, most of the P22 particles are defective. To identify the target of OpdA action, P22 mutants which formed plaques larger than wild-type plaques on an opdA mutant lawn were isolated. Marker rescue experiments using cloned fragments of P22 DNA localized these mutations to a 1-kb fragment. The nucleotide sequence of this fragment and a contiguous region (including all of both P22 gene 7 and gene 14) was determined. The mutations leading to opdA independence affected the region of gene 7 coding for the amino terminus of gp7, a protein required for DNA injection by the phage. Comparison of the nucleotide sequence with the N-terminal amino acid sequence of gp7 suggested that a 20-amino-acid peptide is removed from gp7 during phage development. Further experiments showed that this processing was opdA dependent and rapid (half-life, less than 2 min) and occurred in the absence of other phage proteins. The opdA-independent mutations lead to mutant forms of gp7 which function without processing.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Botstein D., Waddell C. H., King J. Mechanism of head assembly and DNA encapsulation in Salmonella phage p22. I. Genes, proteins, structures and DNA maturation. J Mol Biol. 1973 Nov 15;80(4):669–695. doi: 10.1016/0022-2836(73)90204-0. [DOI] [PubMed] [Google Scholar]
- Brosius J. Superpolylinkers in cloning and expression vectors. DNA. 1989 Dec;8(10):759–777. doi: 10.1089/dna.1989.8.759. [DOI] [PubMed] [Google Scholar]
- Bryant J. L., Jr, King J. DNA injection proteins are targets of acridine-sensitized photoinactivation of bacteriophage P22. J Mol Biol. 1984 Dec 25;180(4):837–863. doi: 10.1016/0022-2836(84)90260-2. [DOI] [PubMed] [Google Scholar]
- Casjens S., Hayden M., Jackson E., Deans R. Additional restriction endonuclease cleavage sites on the bacteriophage P22 genome. J Virol. 1983 Feb;45(2):864–867. doi: 10.1128/jvi.45.2.864-867.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S., King J. P22 morphogenesis. I: Catalytic scaffolding protein in capsid assembly. J Supramol Struct. 1974;2(2-4):202–224. doi: 10.1002/jss.400020215. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chisholm R. L., Deans R. J., Jackson E. N., Jackson D. A., Rutila J. E. A physical gene map of the bacteriophage P22 late region: genetic analysis of cloned fragments of P22 DNA. Virology. 1980 Apr 15;102(1):172–189. doi: 10.1016/0042-6822(80)90079-3. [DOI] [PubMed] [Google Scholar]
- Conlin C. A., Miller C. G. Cloning and nucleotide sequence of opdA, the gene encoding oligopeptidase A in Salmonella typhimurium. J Bacteriol. 1992 Mar;174(5):1631–1640. doi: 10.1128/jb.174.5.1631-1640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlin C. A., Trun N. J., Silhavy T. J., Miller C. G. Escherichia coli prlC encodes an endopeptidase and is homologous to the Salmonella typhimurium opdA gene. J Bacteriol. 1992 Sep;174(18):5881–5887. doi: 10.1128/jb.174.18.5881-5887.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Eppler K., Wyckoff E., Goates J., Parr R., Casjens S. Nucleotide sequence of the bacteriophage P22 genes required for DNA packaging. Virology. 1991 Aug;183(2):519–538. doi: 10.1016/0042-6822(91)90981-g. [DOI] [PubMed] [Google Scholar]
- Gallusser A., Kuhn A. Initial steps in protein membrane insertion. Bacteriophage M13 procoat protein binds to the membrane surface by electrostatic interaction. EMBO J. 1990 Sep;9(9):2723–2729. doi: 10.1002/j.1460-2075.1990.tb07459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer M. S. Uses of the transposon gamma delta in the analysis of cloned genes. Methods Enzymol. 1983;101:362–369. doi: 10.1016/0076-6879(83)01027-7. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W., Casjens S. R. Protein cleavage in bacteriophage lambda tail assembly. Virology. 1974 Sep;61(1):156–159. doi: 10.1016/0042-6822(74)90250-5. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W., Casjens S. R. Protein fusion: a novel reaction in bacteriophage lambda head assembly. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1451–1455. doi: 10.1073/pnas.71.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B., Levine M. Bacteriophage P22 virion protein which performs an essential early function. II. Characterization of the gene 16 function. J Virol. 1975 Dec;16(6):1547–1559. doi: 10.1128/jvi.16.6.1547-1559.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel V. E proteins of bacteriophage P22. I. Identification and ejection from wild-type and defective particles. J Virol. 1977 Jul;23(1):91–97. doi: 10.1128/jvi.23.1.91-97.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita S., Kanegasaki S. Smooth specific phage adsorption: endorhamnosidase activity of tail parts of P22. Biochem Biophys Res Commun. 1973 Nov 16;55(2):403–409. doi: 10.1016/0006-291x(73)91101-7. [DOI] [PubMed] [Google Scholar]
- Jongeneel C. V., Bouvier J., Bairoch A. A unique signature identifies a family of zinc-dependent metallopeptidases. FEBS Lett. 1989 Jan 2;242(2):211–214. doi: 10.1016/0014-5793(89)80471-5. [DOI] [PubMed] [Google Scholar]
- Joshi A., Siddiqui J. Z., Verma M., Chakravorty M. Participation of the host protein(s) in the morphogenesis of bacteriophage P22. Mol Gen Genet. 1982;186(1):44–49. doi: 10.1007/BF00422910. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu L., Whalen W., Das A., Berg C. M. Rapid sequencing of cloned DNA using a transposon for bidirectional priming: sequence of the Escherichia coli K-12 avtA gene. Nucleic Acids Res. 1987 Nov 25;15(22):9461–9469. doi: 10.1093/nar/15.22.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo M. J., Bagga D., Miller C. G. Mutations in rpoA affect expression of anaerobically regulated genes in Salmonella typhimurium. J Bacteriol. 1991 Dec;173(23):7511–7518. doi: 10.1128/jb.173.23.7511-7518.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P., Dev I. K. Degradation of a signal peptide by protease IV and oligopeptidase A. J Bacteriol. 1988 Nov;170(11):5067–5075. doi: 10.1128/jb.170.11.5067-5075.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P., Ray P. H., Dev I. K. Localization and purification of two enzymes from Escherichia coli capable of hydrolyzing a signal peptide. J Biol Chem. 1986 Jan 5;261(1):420–427. [PubMed] [Google Scholar]
- Poteete A. R., King J. Functions of two new genes in Salmonella phage P22 assembly. Virology. 1977 Feb;76(2):725–739. doi: 10.1016/0042-6822(77)90254-9. [DOI] [PubMed] [Google Scholar]
- Ray P., Murialdo H. The role of gene Nu3 in bacteriophage lambda head morphogenesis. Virology. 1975 Mar;64(1):247–263. doi: 10.1016/0042-6822(75)90096-3. [DOI] [PubMed] [Google Scholar]
- Ryu J., Hartin R. J. Quick transformation in Salmonella typhimurium LT2. Biotechniques. 1990 Jan;8(1):43–45. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Sivasubramanian N., Jayaraman R. Mapping of two transcription mutations (tlnI and tlnII) conferring thiolutin resistance, adjacent to dnaZ and rho in Escherichia coli. Mol Gen Genet. 1980;180(3):609–615. doi: 10.1007/BF00268068. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trun N. J., Silhavy T. J. Characterization and in vivo cloning of prlC, a suppressor of signal sequence mutations in Escherichia coli K12. Genetics. 1987 Aug;116(4):513–521. doi: 10.1093/genetics/116.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vimr E. R., Green L., Miller C. G. Oligopeptidase-deficient mutants of Salmonella typhimurium. J Bacteriol. 1983 Mar;153(3):1259–1265. doi: 10.1128/jb.153.3.1259-1265.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr E. R., Miller C. G. Dipeptidyl carboxypeptidase-deficient mutants of Salmonella typhimurium. J Bacteriol. 1983 Mar;153(3):1252–1258. doi: 10.1128/jb.153.3.1252-1258.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston F., Botstein D., Miller J. H. Characterization of amber and ochre suppressors in Salmonella typhimurium. J Bacteriol. 1979 Jan;137(1):433–439. doi: 10.1128/jb.137.1.433-439.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youderian P., Susskind M. M. Identification of the products of bacteriophage P22 genes, including a new late gene. Virology. 1980 Nov;107(1):258–269. doi: 10.1016/0042-6822(80)90291-3. [DOI] [PubMed] [Google Scholar]
- Young B. G. Some phages released from P22-infected salmonella. Virology. 1966 Feb;28(2):249–264. doi: 10.1016/0042-6822(66)90149-8. [DOI] [PubMed] [Google Scholar]