Abstract
FK-506-binding proteins (FKBPs), which in T cells are supposed to mediate the immunosuppressive effects of the compounds FK-506 and rapamycin, have been isolated from Streptomyces chrysomallus, S. hygroscopicus subsp. ascomyceticus, and S. hygroscopicus. The latter two strains are producers of ascomycin (the ethyl analog of FK-506) and rapamycin, respectively. Like the 12-kDa FKBP in eukaryotic organisms such as humans, bovines, and Saccharomyces cerevisiae, or the FKBPs from gram-positive streptomycetes are peptidyl-prolyl-cis-trans isomerases. Inhibition studies using FK-506, rapamycin, or ascomycin, revealed inhibition of the peptidyl-prolyl cis-trans isomerase activity of the proteins at the nanomolar level, which is in the same range as with eukaryotic FKBPs. The M(r)s of the various FKBPs were 13,500 to 15,000, and they had the same pI of approximately 4.5. The N-terminal sequences of the three FKBPs were nearly identical in the first 20 amino acids. The amino acid sequence deduced from the gene sequence of S. chrysomallus gave a polypeptide of 124 amino acids. The homologies to FKBPs from humans, S. cerevisiae, and Neurospora crassa were 38, 39, and 50% identity in relevant positions, respectively. Significant homology of 38% was also seen with the C-terminal halves of bacterial protein surface antigens like the Mip protein of Legionella pneumophila and the 27-kDa Mip-like protein of Chlamydia trachomatis. In addition, two more open reading frames in Pseudomonas aeruginosa and Neisseria meningitidis of unknown function show regions of homology to the S. chrysomallus FKBP. In contrast to fungi, streptomycetes are resistant to macrolactones. Ascomycin-producing S. hygroscopicus subsp. ascomyceticus excretes the compound almost quantitatively into medium, which indicates that the organism has an efficient self-protection mechanism against its own secondary metabolite.
Full text
PDF

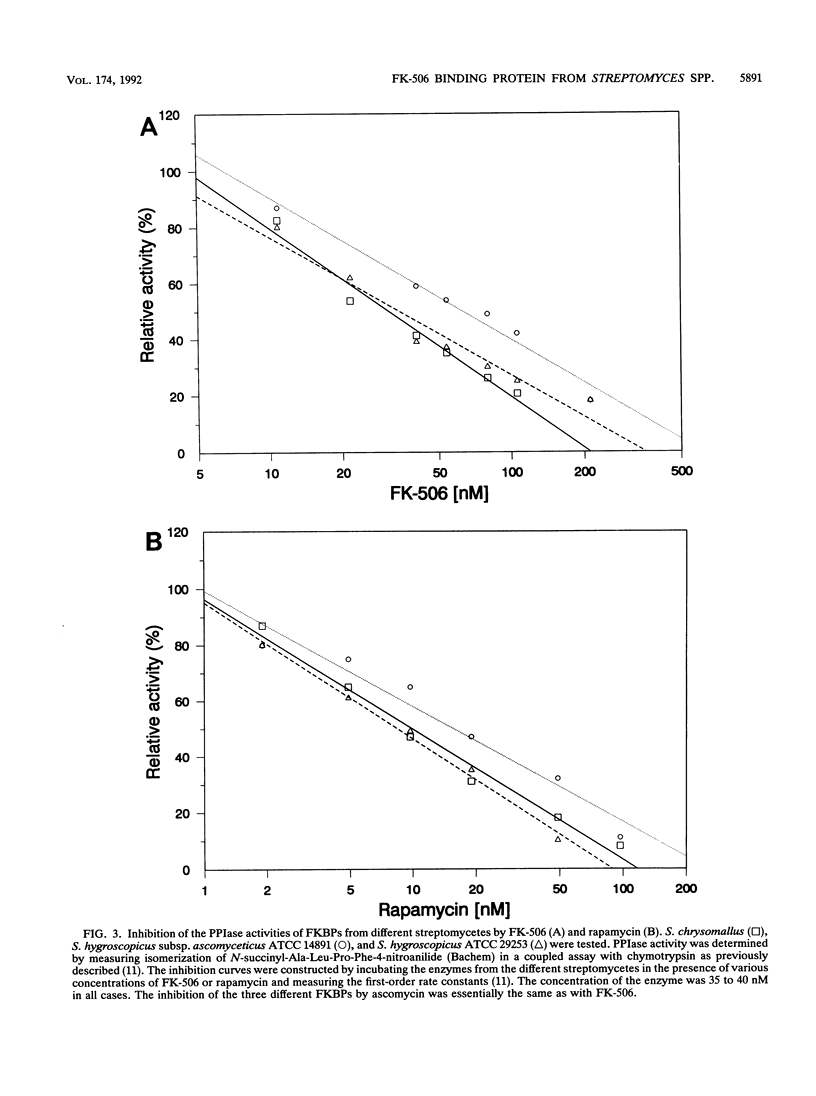


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAI T., KOUAMA Y., SUENAGA T., HONDA H. Ascomycin, an antifungal antibiotic. J Antibiot (Tokyo) 1962 Nov;15:231–232. [PubMed] [Google Scholar]
- Bierer B. E., Mattila P. S., Standaert R. F., Herzenberg L. A., Burakoff S. J., Crabtree G., Schreiber S. L. Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9231–9235. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer B. E., Somers P. K., Wandless T. J., Burakoff S. J., Schreiber S. L. Probing immunosuppressant action with a nonnatural immunophilin ligand. Science. 1990 Oct 26;250(4980):556–559. doi: 10.1126/science.1700475. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cianciotto N. P., Eisenstein B. I., Mody C. H., Toews G. B., Engleberg N. C. A Legionella pneumophila gene encoding a species-specific surface protein potentiates initiation of intracellular infection. Infect Immun. 1989 Apr;57(4):1255–1262. doi: 10.1128/iai.57.4.1255-1262.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley N. J., Baker E. K., Stamnes M. A., Zuker C. S. The cyclophilin homolog ninaA is required in the secretory pathway. Cell. 1991 Oct 18;67(2):255–263. doi: 10.1016/0092-8674(91)90177-z. [DOI] [PubMed] [Google Scholar]
- Cundliffe E. How antibiotic-producing organisms avoid suicide. Annu Rev Microbiol. 1989;43:207–233. doi: 10.1146/annurev.mi.43.100189.001231. [DOI] [PubMed] [Google Scholar]
- Dumont F. J., Melino M. R., Staruch M. J., Koprak S. L., Fischer P. A., Sigal N. H. The immunosuppressive macrolides FK-506 and rapamycin act as reciprocal antagonists in murine T cells. J Immunol. 1990 Feb 15;144(4):1418–1424. [PubMed] [Google Scholar]
- Dumont F. J., Staruch M. J., Koprak S. L., Melino M. R., Sigal N. H. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol. 1990 Jan 1;144(1):251–258. [PubMed] [Google Scholar]
- Fischer G., Bang H., Ludwig B., Mann K., Hacker J. Mip protein of Legionella pneumophila exhibits peptidyl-prolyl-cis/trans isomerase (PPlase) activity. Mol Microbiol. 1992 May;6(10):1375–1383. doi: 10.1111/j.1365-2958.1992.tb00858.x. [DOI] [PubMed] [Google Scholar]
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989 Feb 2;337(6206):476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Galat A., Uehling D. E., Schreiber S. L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989 Oct 26;341(6244):758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- Harrison R. K., Stein R. L. Substrate specificities of the peptidyl prolyl cis-trans isomerase activities of cyclophilin and FK-506 binding protein: evidence for the existence of a family of distinct enzymes. Biochemistry. 1990 Apr 24;29(16):3813–3816. doi: 10.1021/bi00468a001. [DOI] [PubMed] [Google Scholar]
- Hatanaka H., Kino T., Miyata S., Inamura N., Kuroda A., Goto T., Tanaka H., Okuhara M. FR-900520 and FR-900523, novel immunosuppressants isolated from a Streptomyces. II. Fermentation, isolation and physico-chemical and biological characteristics. J Antibiot (Tokyo) 1988 Nov;41(11):1592–1601. doi: 10.7164/antibiotics.41.1592. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A. The Leeuwenhoek lecture, 1987. Towards an understanding of gene switching in Streptomyces, the basis of sporulation and antibiotic production. Proc R Soc Lond B Biol Sci. 1988 Nov 22;235(1279):121–138. doi: 10.1098/rspb.1988.0067. [DOI] [PubMed] [Google Scholar]
- Hultsch T., Albers M. W., Schreiber S. L., Hohman R. J. Immunophilin ligands demonstrate common features of signal transduction leading to exocytosis or transcription. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6229–6233. doi: 10.1073/pnas.88.14.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J., Chu L., Kitano K., DeVault J. D., Kimbara K., Chakrabarty A. M., Misra T. K. Nucleotide sequence of a regulatory region controlling alginate synthesis in Pseudomonas aeruginosa: characterization of the algR2 gene. Gene. 1989 Dec 7;84(1):31–38. doi: 10.1016/0378-1119(89)90136-4. [DOI] [PubMed] [Google Scholar]
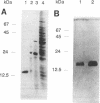
- Keller U., Krengel U., Haese A. Genetic analysis in Streptomyces chrysomallus. J Gen Microbiol. 1985 May;131(5):1181–1191. doi: 10.1099/00221287-131-5-1181. [DOI] [PubMed] [Google Scholar]
- Keller U., Schlumbohm W. Purification and characterization of actinomycin synthetase I, a 4-methyl-3-hydroxyanthranilic acid-AMP ligase from Streptomyces chrysomallus. J Biol Chem. 1992 Jun 15;267(17):11745–11752. [PubMed] [Google Scholar]
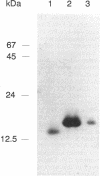
- Kino T., Hatanaka H., Hashimoto M., Nishiyama M., Goto T., Okuhara M., Kohsaka M., Aoki H., Imanaka H. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot (Tokyo) 1987 Sep;40(9):1249–1255. doi: 10.7164/antibiotics.40.1249. [DOI] [PubMed] [Google Scholar]
- Koltin Y., Faucette L., Bergsma D. J., Levy M. A., Cafferkey R., Koser P. L., Johnson R. K., Livi G. P. Rapamycin sensitivity in Saccharomyces cerevisiae is mediated by a peptidyl-prolyl cis-trans isomerase related to human FK506-binding protein. Mol Cell Biol. 1991 Mar;11(3):1718–1723. doi: 10.1128/mcb.11.3.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Lundemose A. G., Birkelund S., Fey S. J., Larsen P. M., Christiansen G. Chlamydia trachomatis contains a protein similar to the Legionella pneumophila mip gene product. Mol Microbiol. 1991 Jan;5(1):109–115. doi: 10.1111/j.1365-2958.1991.tb01831.x. [DOI] [PubMed] [Google Scholar]
- Mattila P. S., Ullman K. S., Fiering S., Emmel E. A., McCutcheon M., Crabtree G. R., Herzenberg L. A. The actions of cyclosporin A and FK506 suggest a novel step in the activation of T lymphocytes. EMBO J. 1990 Dec;9(13):4425–4433. doi: 10.1002/j.1460-2075.1990.tb07893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Perry A. C., Nicolson I. J., Saunders J. R. Neisseria meningitidis C114 contains silent, truncated pilin genes that are homologous to Neisseria gonorrhoeae pil sequences. J Bacteriol. 1988 Apr;170(4):1691–1697. doi: 10.1128/jb.170.4.1691-1697.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson B. A., Gotschlich E. C. Neisseria meningitidis encodes an FK506-inhibitable rotamase. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1164–1168. doi: 10.1073/pnas.89.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991 Jan 18;251(4991):283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- Sehgal S. N., Baker H., Vézina C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo) 1975 Oct;28(10):727–732. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- Siekierka J. J., Hung S. H., Poe M., Lin C. S., Sigal N. H. A cytosolic binding protein for the immunosuppressant FK506 has peptidyl-prolyl isomerase activity but is distinct from cyclophilin. Nature. 1989 Oct 26;341(6244):755–757. doi: 10.1038/341755a0. [DOI] [PubMed] [Google Scholar]
- Siekierka J. J., Wiederrecht G., Greulich H., Boulton D., Hung S. H., Cryan J., Hodges P. J., Sigal N. H. The cytosolic-binding protein for the immunosuppressant FK-506 is both a ubiquitous and highly conserved peptidyl-prolyl cis-trans isomerase. J Biol Chem. 1990 Dec 5;265(34):21011–21015. [PubMed] [Google Scholar]
- Stamnes M. A., Shieh B. H., Chuman L., Harris G. L., Zuker C. S. The cyclophilin homolog ninaA is a tissue-specific integral membrane protein required for the proper synthesis of a subset of Drosophila rhodopsins. Cell. 1991 Apr 19;65(2):219–227. doi: 10.1016/0092-8674(91)90156-s. [DOI] [PubMed] [Google Scholar]
- Standaert R. F., Galat A., Verdine G. L., Schreiber S. L. Molecular cloning and overexpression of the human FK506-binding protein FKBP. Nature. 1990 Aug 16;346(6285):671–674. doi: 10.1038/346671a0. [DOI] [PubMed] [Google Scholar]
- Tocci M. J., Matkovich D. A., Collier K. A., Kwok P., Dumont F., Lin S., Degudicibus S., Siekierka J. J., Chin J., Hutchinson N. I. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J Immunol. 1989 Jul 15;143(2):718–726. [PubMed] [Google Scholar]
- Tropschug M., Wachter E., Mayer S., Schönbrunner E. R., Schmid F. X. Isolation and sequence of an FK506-binding protein from N. crassa which catalyses protein folding. Nature. 1990 Aug 16;346(6285):674–677. doi: 10.1038/346674a0. [DOI] [PubMed] [Google Scholar]