Abstract
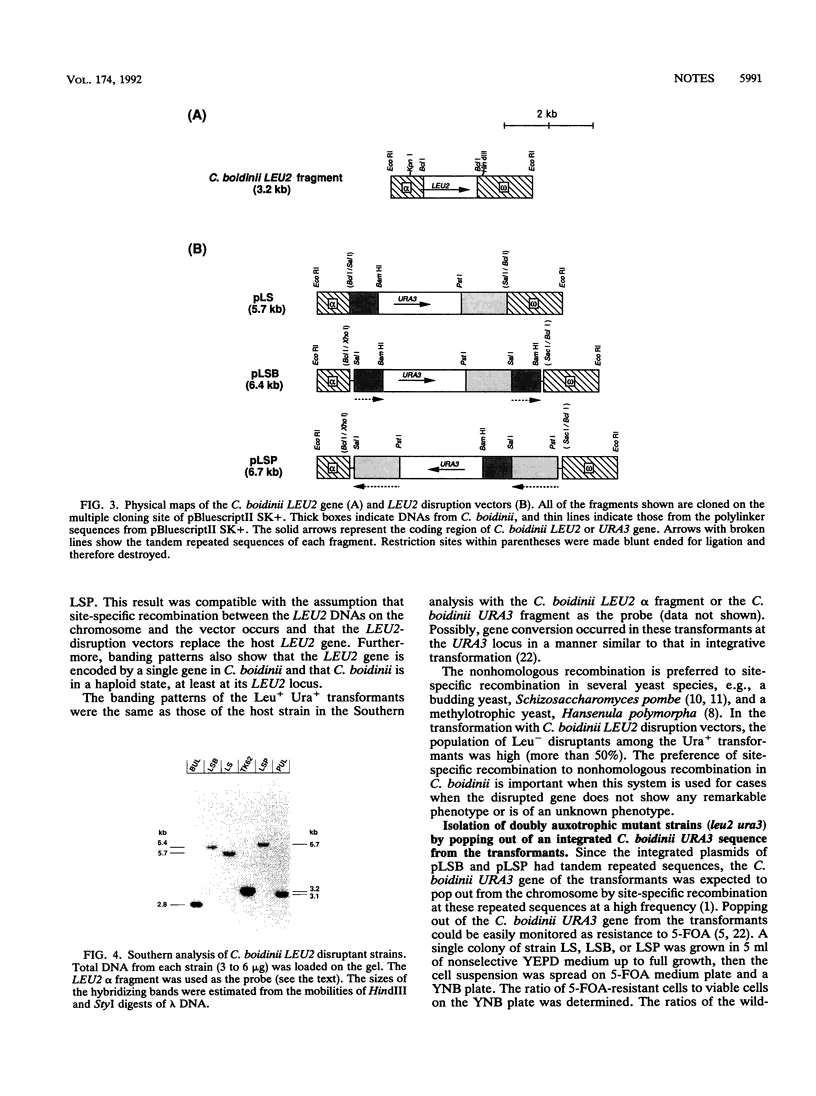
A model system for one-step gene disruption for an asporogenous methylotrophic yeast, Candida boidinii, is described. In this system, the 3-isopropylmalate dehydrogenase gene (C. boidinii LEU2) was selected as the target gene for disruption to derive new host strains for transformation. First, the C. boidinii LEU2 gene was cloned, and its complete nucleotide sequence was determined. Next, the LEU2 disruption vectors, which had the C. boidinii URA3 gene as the selectable marker, were constructed. Of the Ura+ transformants obtained with these plasmids, more than half showed a Leu- phenotype. Finally, the double-marker strains of C. boidinii were derived. When vectors with repeated flanking sequences of the C. boidinii URA3 gene were used for gene disruption, Leu- Ura+ transformants changed spontaneously to a Leu- Ura- phenotype ca. 100 times more frequently than they did when plasmids without the repeated sequences were used. Southern analysis showed that these events included a one-step gene disruption and a subsequent popping out of the C. boidinii URA3 sequence from the transformant chromosome.
Full text
PDF




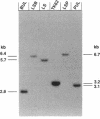
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alani E., Cao L., Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987 Aug;116(4):541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadis A., Hsu Y. P., Kohlhaw G. B., Schimmel P. Nucleotide sequence of yeast LEU2 shows 5'-noncoding region has sequences cognate to leucine. Cell. 1982 Dec;31(2 Pt 1):319–325. doi: 10.1016/0092-8674(82)90125-8. [DOI] [PubMed] [Google Scholar]
- Bellion E., Goodman J. M. Proton ionophores prevent assembly of a peroxisomal protein. Cell. 1987 Jan 16;48(1):165–173. doi: 10.1016/0092-8674(87)90367-9. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., LaCroute F., Fink G. R. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Cryer D. R., Eccleshall R., Marmur J. Isolation of yeast DNA. Methods Cell Biol. 1975;12:39–44. doi: 10.1016/s0091-679x(08)60950-4. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garrard L. J., Goodman J. M. Two genes encode the major membrane-associated protein of methanol-induced peroxisomes from Candida boidinii. J Biol Chem. 1989 Aug 15;264(23):13929–13937. [PubMed] [Google Scholar]
- Gellissen G., Janowicz Z. A., Merckelbach A., Piontek M., Keup P., Weydemann U., Hollenberg C. P., Strasser A. W. Heterologous gene expression in Hansenula polymorpha: efficient secretion of glucoamylase. Biotechnology (N Y) 1991 Mar;9(3):291–295. doi: 10.1038/nbt0391-291. [DOI] [PubMed] [Google Scholar]
- Grimm C., Kohli J., Murray J., Maundrell K. Genetic engineering of Schizosaccharomyces pombe: a system for gene disruption and replacement using the ura4 gene as a selectable marker. Mol Gen Genet. 1988 Dec;215(1):81–86. doi: 10.1007/BF00331307. [DOI] [PubMed] [Google Scholar]
- Grimm C., Kohli J. Observations on integrative transformation in Schizosaccharomyces pombe. Mol Gen Genet. 1988 Dec;215(1):87–93. doi: 10.1007/BF00331308. [DOI] [PubMed] [Google Scholar]
- Hamasawa K., Kobayashi Y., Harada S., Yoda K., Yamasaki M., Tamura G. Molecular cloning and nucleotide sequence of the 3-isopropylmalate dehydrogenase gene of Candida utilis. J Gen Microbiol. 1987 Apr;133(4):1089–1097. doi: 10.1099/00221287-133-4-1089. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y. P., Schimmel P. Yeast LEU1. Repression of mRNA levels by leucine and relationship of 5'-noncoding region to that of LEU2. J Biol Chem. 1984 Mar 25;259(6):3714–3719. [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R., Miller S. M., Kurtz M. B., Kirsch D. R. Directed mutagenesis in Candida albicans: one-step gene disruption to isolate ura3 mutants. Mol Cell Biol. 1987 Jan;7(1):199–208. doi: 10.1128/mcb.7.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picataggio S., Deanda K., Mielenz J. Determination of Candida tropicalis acyl coenzyme A oxidase isozyme function by sequential gene disruption. Mol Cell Biol. 1991 Sep;11(9):4333–4339. doi: 10.1128/mcb.11.9.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribnow D. Bacteriophage T7 early promoters: nucleotide sequences of two RNA polymerase binding sites. J Mol Biol. 1975 Dec 15;99(3):419–443. doi: 10.1016/s0022-2836(75)80136-7. [DOI] [PubMed] [Google Scholar]
- Ratzkin B., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Feb;74(2):487–491. doi: 10.1073/pnas.74.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- Sakai Y., Kazarimoto T., Tani Y. Transformation system for an asporogenous methylotrophic yeast, Candida boidinii: cloning of the orotidine-5'-phosphate decarboxylase gene (URA3), isolation of uracil auxotrophic mutants, and use of the mutants for integrative transformation. J Bacteriol. 1991 Dec;173(23):7458–7463. doi: 10.1128/jb.173.23.7458-7463.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y., Tani Y. Production of Formaldehyde by Detergent-Treated Cells of a Methanol Yeast, Candida boidinii S2 Mutant Strain AOU-1. Appl Environ Microbiol. 1988 Feb;54(2):485–489. doi: 10.1128/aem.54.2.485-489.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takagi M., Kobayashi N., Sugimoto M., Fujii T., Watari J., Yano K. Nucleotide sequencing analysis of a LEU gene of Candida maltosa which complements leuB mutation of Escherichia coli and leu2 mutation of Saccharomyces cerevisiae. Curr Genet. 1987;11(6-7):451–457. doi: 10.1007/BF00384606. [DOI] [PubMed] [Google Scholar]
- Tani Y. Production of useful chemicals by methylotrophs. Biotechnology. 1991;18:253–270. doi: 10.1016/b978-0-7506-9188-8.50018-8. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982 Mar;28(3):563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]