Abstract
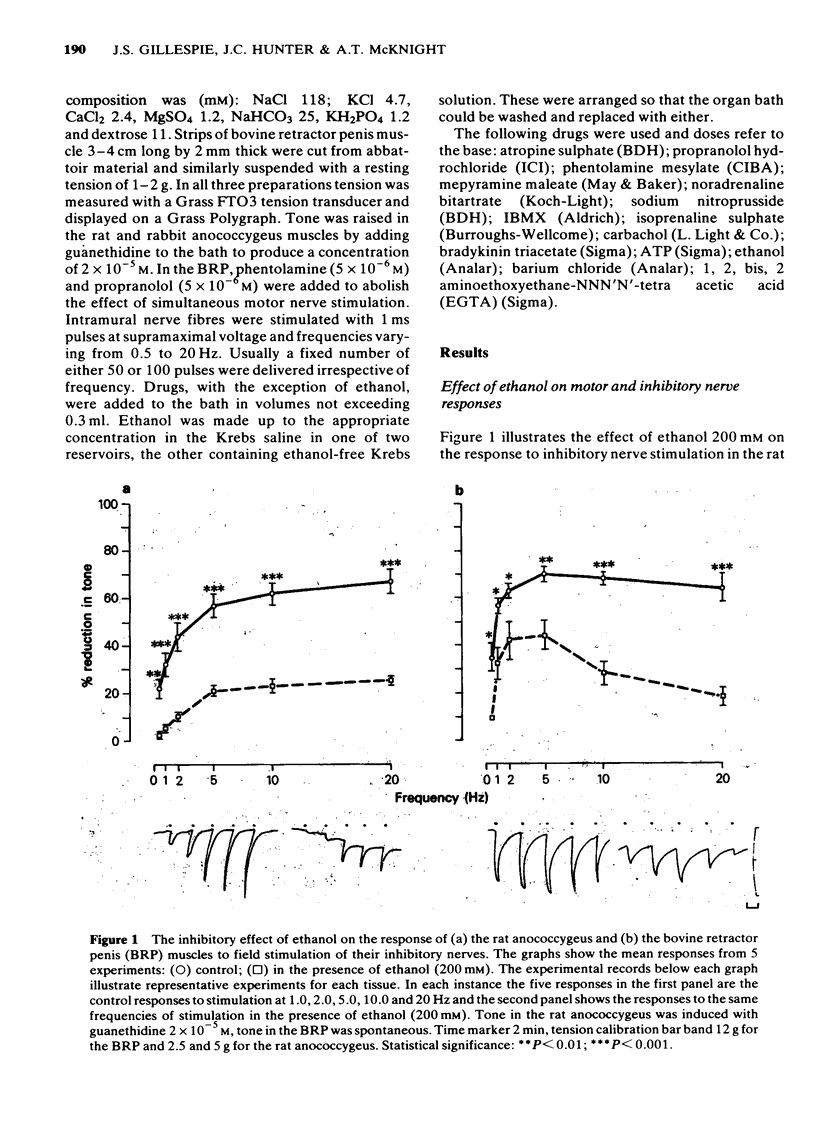
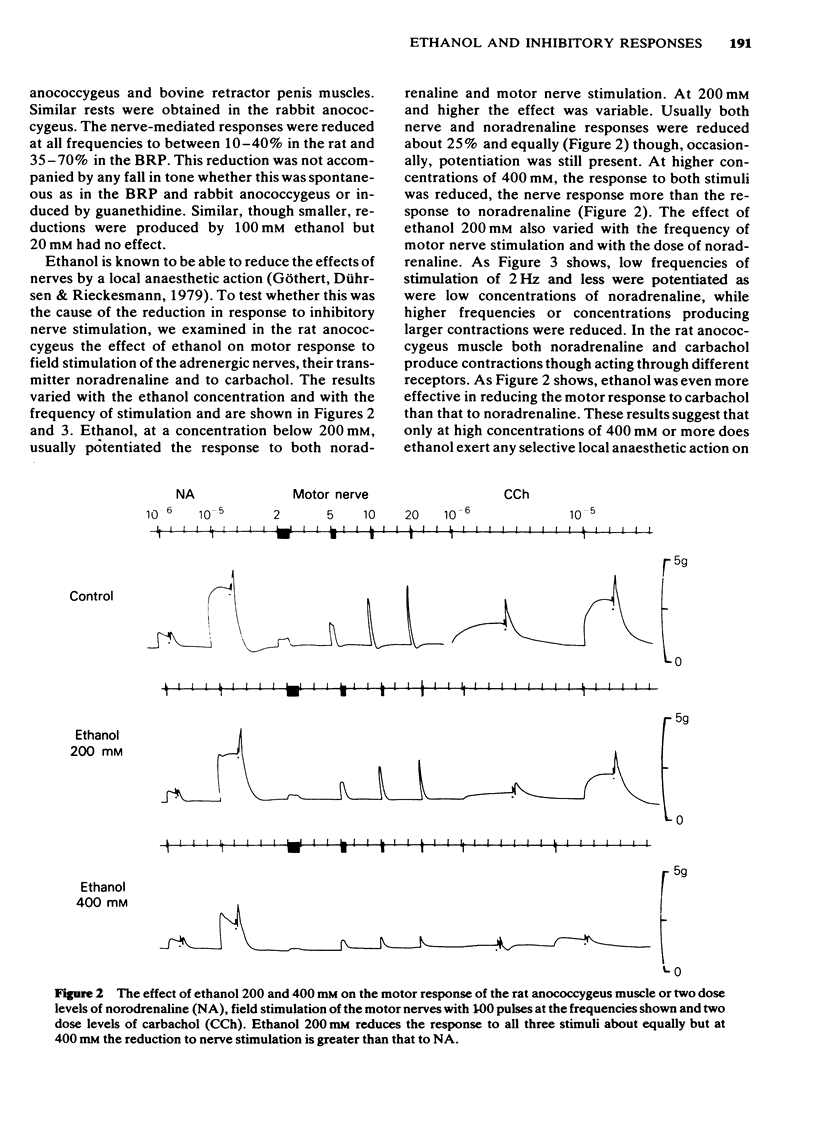
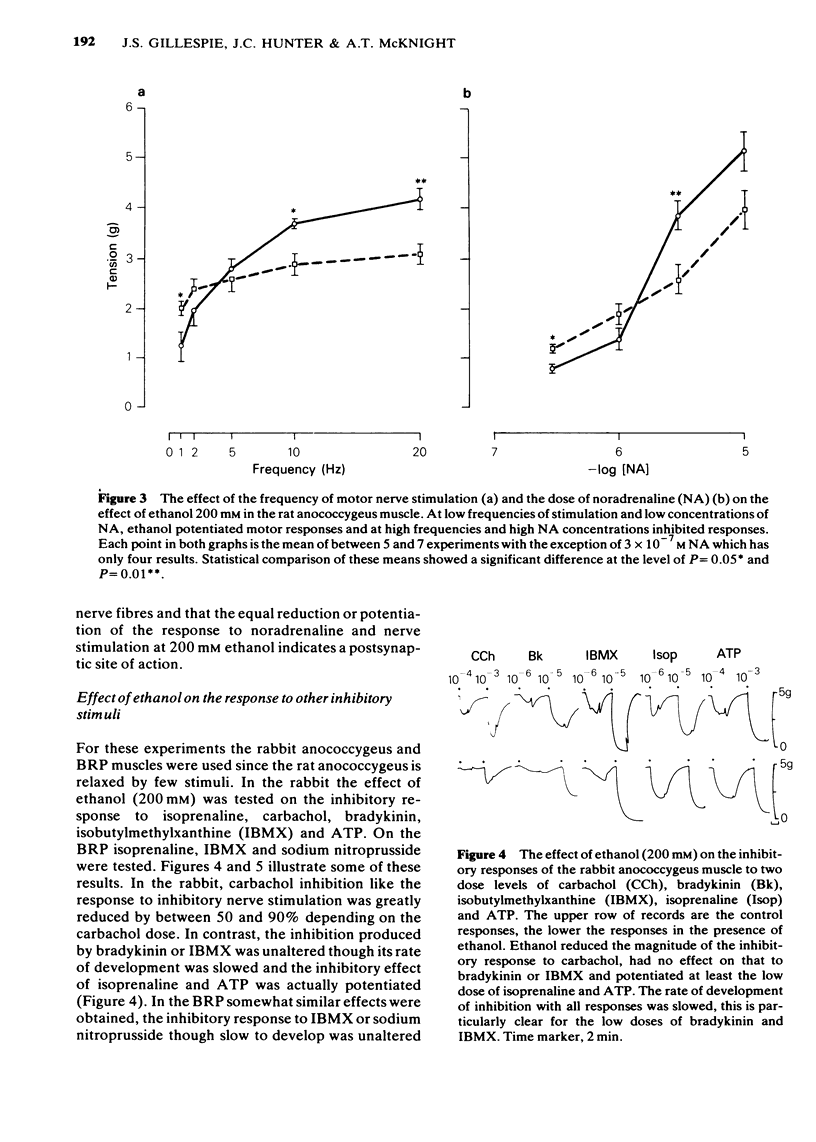
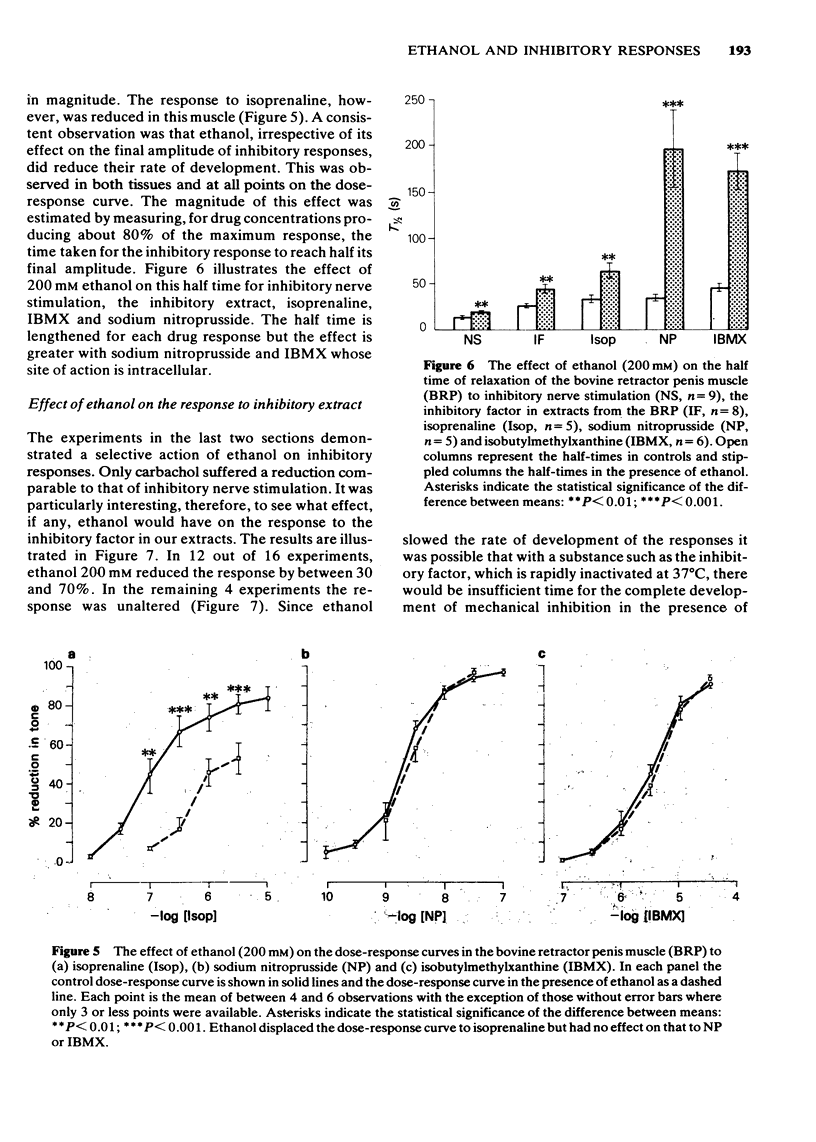
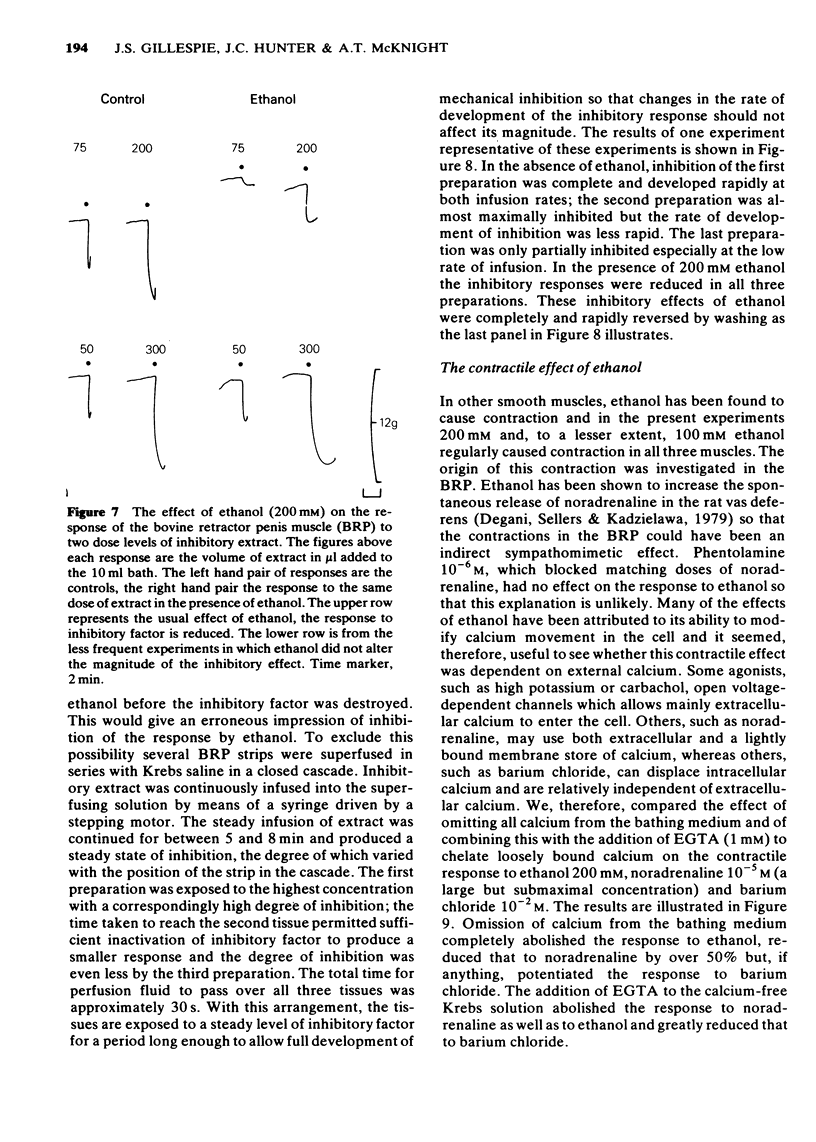
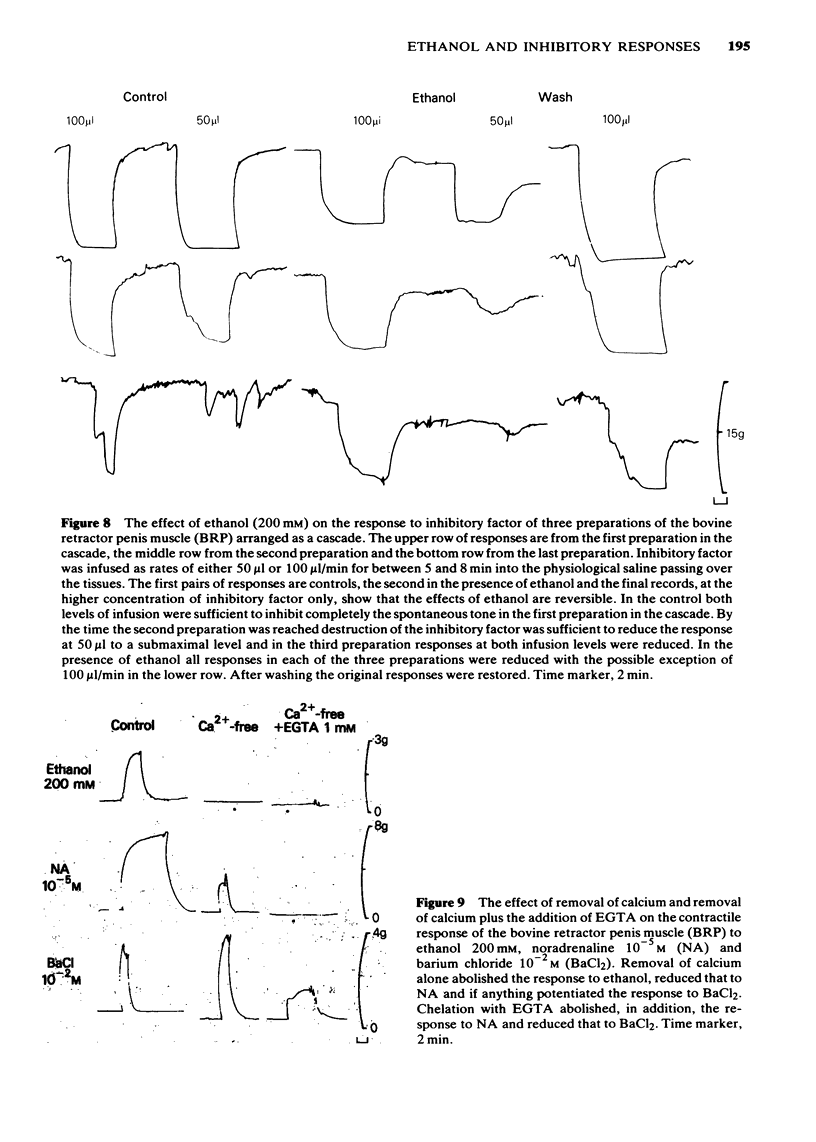
1 Ethanol (200 mM) reduced the response to inhibitory nerve stimulation in the rat and rabbit anococcygeus and the bovine retractor penis (BRP) muscles. Ethanol also reduced the response to the inhibitory extract from the BRP consistent with the inhibitory factor in these extracts playing some part in the response to inhibitory nerve stimulation. 2 Ethanol's effect on the response to other inhibitory stimuli was examined in the rabbit anococcygeus and the BRP. In the anococcygeus the response to carbachol was reduced, to bradykinin and isobutylmethylxanthine (IBMX) unaltered, and to isoprenaline and adenosine 5'-triphosphate (ATP) potentiated. In the BRP responses to IBMX and sodium nitroprusside were unaltered but in this tissue the response to isoprenaline was reduced. Ethanol's ability to reduce inhibitory responses is, therefore, selective and confined to inhibitory nerve stimulation, inhibitory extract, carbachol, and, in the BRP, isoprenaline. 3 Ethanol reduced the rate of development of inhibition even where the magnitude of the inhibitory response was unaltered. 4 In the rat anococcygeus, ethanol (200 mM) potentiated the response to motor nerve stimulation and to noradrenaline (NA) at low frequencies and low concentrations respectively. Higher ethanol concentrations (400 mM) reduced the response to both motor nerve stimulation and NA. The motor response to carbachol was also reduced. 5 Ethanol (200 mM) itself caused an easily reversible contraction in all three tissues. This was not due to the release of NA but was highly sensitive to the removal of external calcium from the medium. 6 A unified explanation of these varied effects of ethanol based on a reduction in membrane binding of calcium and a reduced efficiency of receptor coupling is suggested.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altura B. M., Edgarian H., Altura B. T. Differential effects of ethanol and mannitol on contraction of arterial smooth muscle. J Pharmacol Exp Ther. 1976 May;197(2):352–361. [PubMed] [Google Scholar]
- Altura B. M., Edgarian H. Ethanol-prostaglandin interactions in contraction of vascular smooth muscle. Proc Soc Exp Biol Med. 1976 Jul;152(3):334–336. doi: 10.3181/00379727-152-39391. [DOI] [PubMed] [Google Scholar]
- Altura B. M., Ogunkoya A., Gebrewold A., Altura B. T. Effects of ethanol on terminal arterioles and muscular venules: direct observations on the microcirculation. J Cardiovasc Pharmacol. 1979 Jan-Feb;1(1):97–113. doi: 10.1097/00005344-197901000-00010. [DOI] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Martin W. The inhibitory material in extracts from the bovine retractor penis muscle is not an adenine nucleotide. Br J Pharmacol. 1979 Nov;67(3):327–328. doi: 10.1111/j.1476-5381.1979.tb08683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. H., Goldstein D. B. Drug tolerance in biomembranes: a spin label study of the effects of ethanol. Science. 1977 May 6;196(4290):684–685. doi: 10.1126/science.193186. [DOI] [PubMed] [Google Scholar]
- Clement J. G. Ethanol potentiation of choline, acetylcholine, carbachol and phenyltrimethylammonium contractions in the chick biventer cervicis muscle. Eur J Pharmacol. 1980 Jan 25;61(2):195–198. doi: 10.1016/0014-2999(80)90165-x. [DOI] [PubMed] [Google Scholar]
- Creed K. E., Gillespie J. S., McCaffery H. The rabbit anococcygeus muscle and its response to field stimulation and to some drugs. J Physiol. 1977 Dec;273(1):121–135. doi: 10.1113/jphysiol.1977.sp012085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degani N. C., Sellers E. M., Kadzielawa K. Ethanol-induced spontaneous norepinephrine release from the rat vas deferens. J Pharmacol Exp Ther. 1979 Jul;210(1):22–26. [PubMed] [Google Scholar]
- Fewings J. D., Hanna M. J., Walsh J. A., Whelan R. F. The effects of ethyl alcohol on the blood vessels of the hand and forearm in man. Br J Pharmacol Chemother. 1966 May;27(1):93–106. doi: 10.1111/j.1476-5381.1966.tb01644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W. The effect of methyl, ethyl and n-propyl alcohol on neuromuscular transmission in the rat. J Pharmacol Exp Ther. 1965 Nov;150(2):236–243. [PubMed] [Google Scholar]
- Gillespie J. S., Martin W. A smooth muscle inhibitory material from the bovine retractor penis and rat anococcygeus muscles. J Physiol. 1980 Dec;309:55–64. doi: 10.1113/jphysiol.1980.sp013493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., McKnight A. T. The actions of some vasoactive polypeptides and their antagonists on the anococcygeus muscle. Br J Pharmacol. 1978 Feb;62(2):267–274. doi: 10.1111/j.1476-5381.1978.tb08455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S. The rat anococcygeus muscle and its response to nerve stimulation and to some drugs. Br J Pharmacol. 1972 Jul;45(3):404–416. doi: 10.1111/j.1476-5381.1972.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göthert M., Dührsen U., Rieckesmann J. M. Ethanol, anaesthetics and other lipophilic drugs preferentially inhibit 5-hydroxytryptamine- and acetylcholine-induced noradrenaline release from sympathetic nerves. Arch Int Pharmacodyn Ther. 1979 Dec;242(2):196–209. [PubMed] [Google Scholar]
- HURWITZ L., BATTLE F., WEISS G. B. Action of the calcium antagonists cocaine and ethanol on contraction and potassium efflux of smooth muscle. J Gen Physiol. 1962 Nov;46:315–332. doi: 10.1085/jgp.46.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. A., Hood W. F. Inhibition of synaptosomal calcium uptake by ethanol. J Pharmacol Exp Ther. 1980 Jun;213(3):562–568. [PubMed] [Google Scholar]
- Kalsner S. The potentiating effects of ethanol on response of aortic strips to stimulant drugs. J Pharm Pharmacol. 1970 Nov;22(11):877–879. doi: 10.1111/j.2042-7158.1970.tb08466.x. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Alcohol, aldehydes, and biogenic amines. Ann N Y Acad Sci. 1973 Apr 30;215:84–88. doi: 10.1111/j.1749-6632.1973.tb28252.x. [DOI] [PubMed] [Google Scholar]
- Mayer J. M., Khanna J. M., Kalant H. A role for calcium in the acute and chronic actions of ethanol in vitro. Eur J Pharmacol. 1980 Nov 21;68(2):223–227. doi: 10.1016/0014-2999(80)90328-3. [DOI] [PubMed] [Google Scholar]
- Reitz R. C. Effect of chronic ethanol ingestion on membrane lipids. Proc West Pharmacol Soc. 1980;23:441–448. [PubMed] [Google Scholar]
- Ross D. H. Selective action of alcohols on cerebral calcium levels. Ann N Y Acad Sci. 1976;273:280–294. doi: 10.1111/j.1749-6632.1976.tb52891.x. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Tabakoff B., Hoffman P. L. Development of functional dependence on ethanol in dopaminergic systems. J Pharmacol Exp Ther. 1979 Feb;208(2):216–222. [PubMed] [Google Scholar]
- Turlapaty P. D., Altura B. T., Altura B. M. Ethanol reduces Ca2+ concentrations in arterial and venous smooth muscle. Experientia. 1979 May 15;35(5):639–640. doi: 10.1007/BF01960370. [DOI] [PubMed] [Google Scholar]
- Turlapaty P. D., Altura B. T., Altura B. M. Interactions of Tris buffer and ethanol on agonist-induced responses of vascular smooth muscle and on calcium-45 uptake. J Pharmacol Exp Ther. 1979 Oct;211(1):59–67. [PubMed] [Google Scholar]
- Vallières J., Scarpa A., Somlyo A. P. Subcellular fractions of smooth muscle. Isolation, substrate utilization and Ca++ transport by main pulmonary artery and mesenteric vein mitochondria. Arch Biochem Biophys. 1975 Oct;170(2):659–669. doi: 10.1016/0003-9861(75)90162-9. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Aaronson P., Loutzenhiser R., Meisheri K. Ca2+ movements in smooth muscle. Chest. 1980 Jul;78(1 Suppl):157–165. doi: 10.1378/chest.78.1_supplement.157. [DOI] [PubMed] [Google Scholar]


