Abstract
1 Secretion of catecholamines (CA) evoked by ouabain, chlormadinone acetate (CMA), phenoxybenzamine (Pbz) and vanadate, four agents known to inhibit Na+, K+-dependent Mg2+-activated adenosine triphosphatase (ATPase) activity has been studied in suspensions of bovine isolated adrenal medullary cells.
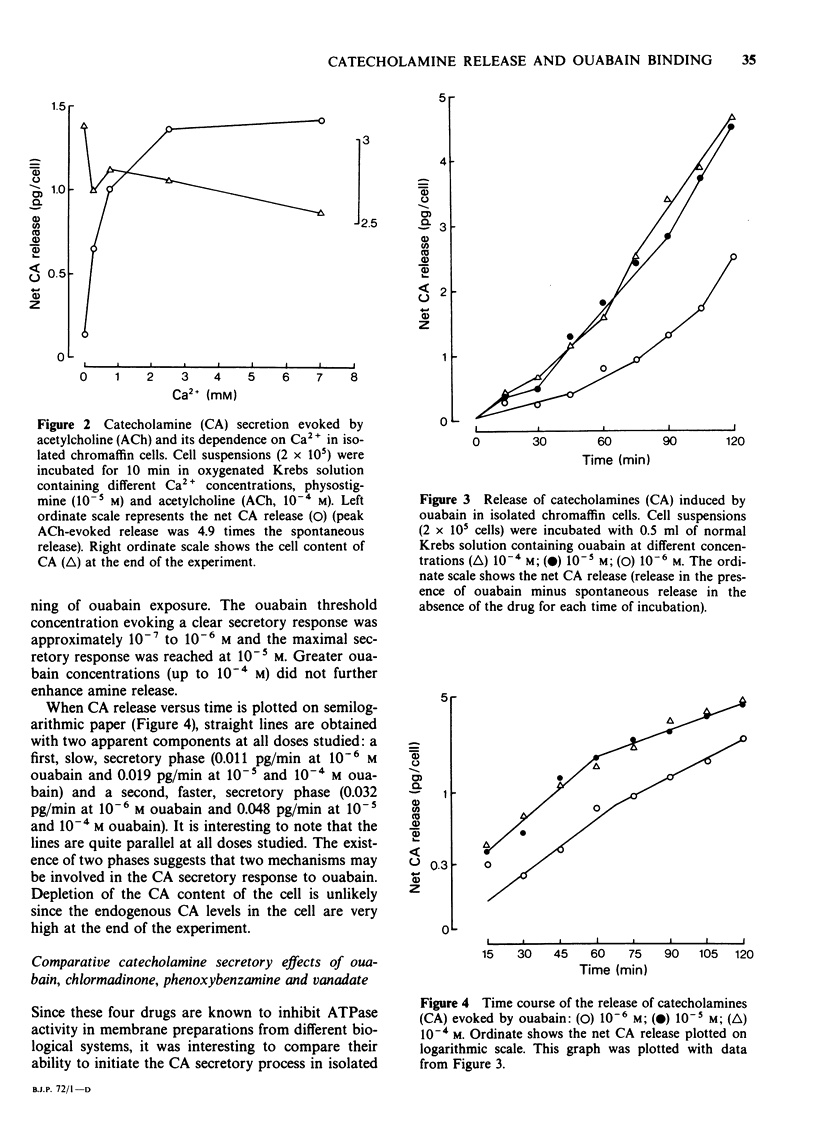
2 Acetylcholine (ACh) evoked a 5 fold increase of the basal CA secretion from isolated cells suspended in oxygenated Krebs-bicarbonate solution kept at 27°C. Secretion was antagonized by Ca2+-deprivation or hexamethonium, indicating good functional viability of the cells.
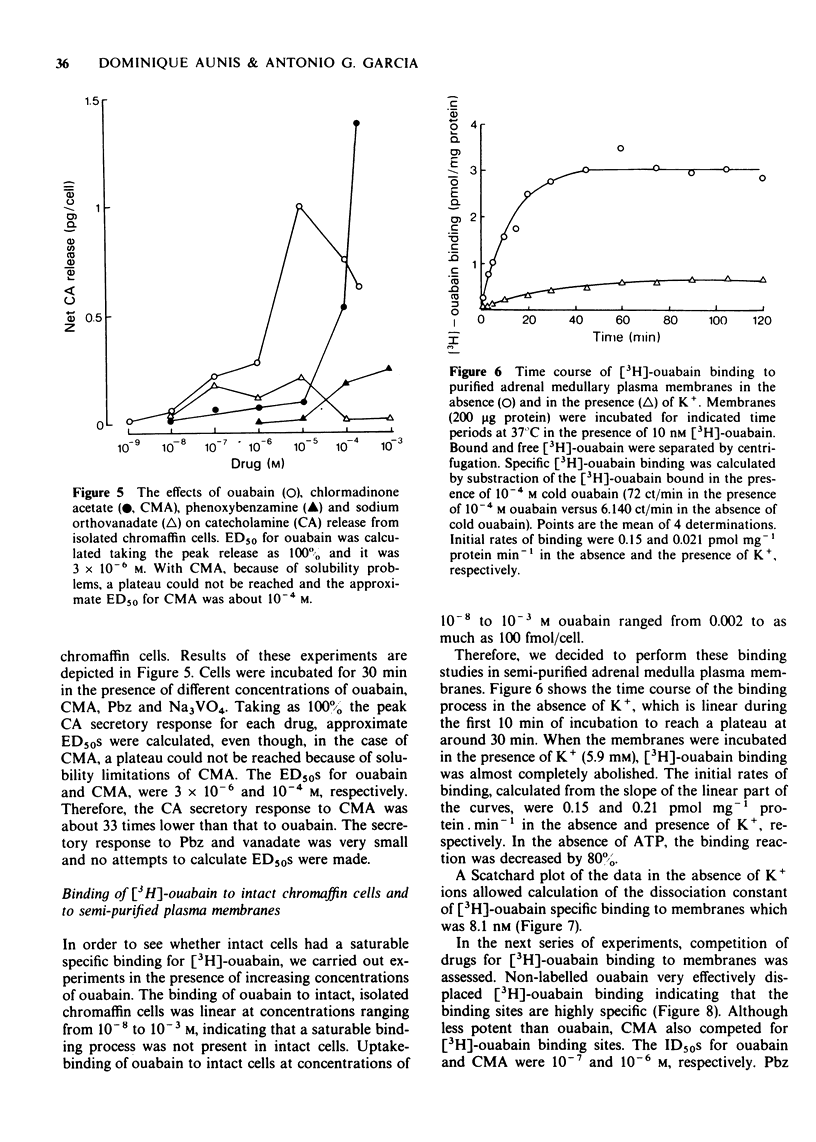
3 Ouabain (10-7 to 10-4 M) evoked a progressive, dose-dependent release of CA from cell suspensions. Study of the time course of the secretory response for 2 h allowed the separation of two components in the secretory response at all doses studied: a slow initial component (0.011 pg/min CA) and a second faster component (0.032 pg/min CA).
4 CMA evoked a clear-cut CA secretory response. The ED50 for CMA was 10-4 M, as compared to 3 × 10-6 M for ouabain. Pbz and vanadate did not induce CA release.
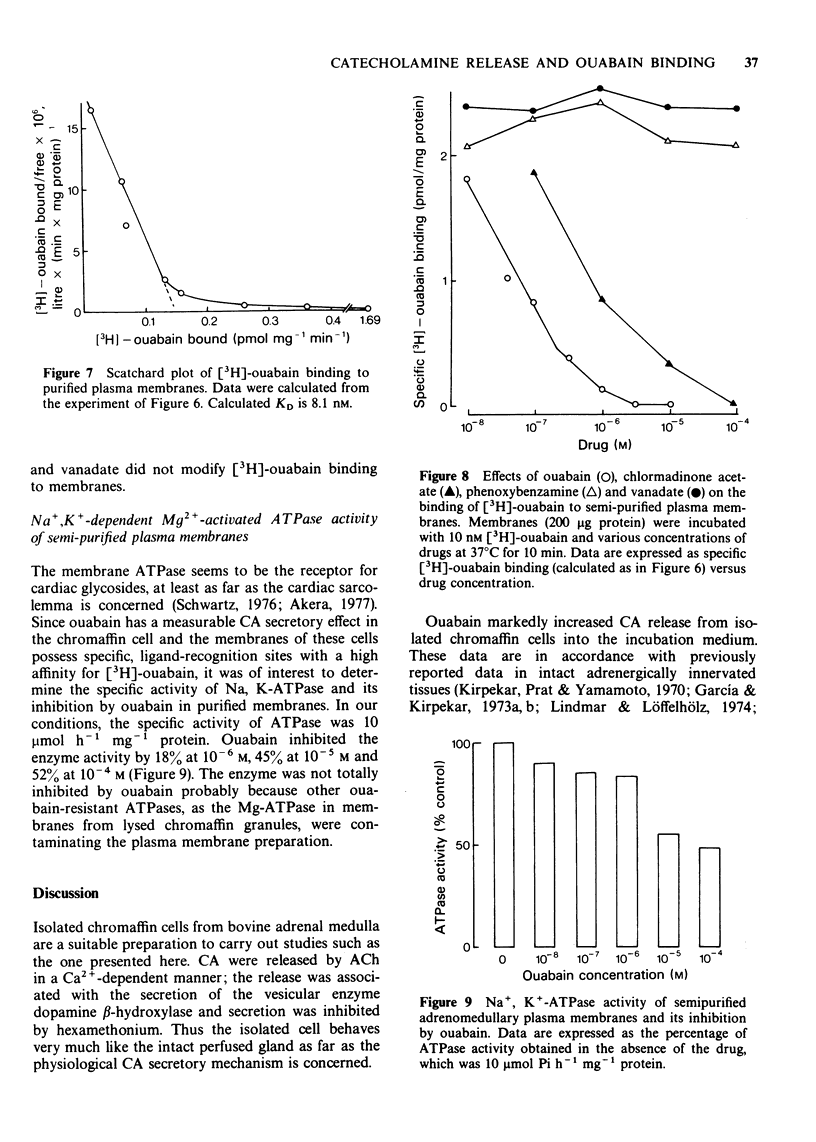
5 [3H]-ouabain was taken up and bound to intact isolated cells by a non-saturable binding process. However, in semi-purified plasma membranes from bovine adrenal medulla a saturable specific [3H]-ouabain binding process was observed with a KD of 8.1 nM. Binding to the membranes was ATP-dependent and antagonized by K+.
6 [3H]-ouabain specific binding to membranes was antagonized by ouabain and CMA, but not by Pbz or vanadate; the ID50 for ouabain and CMA were 10-6 and 10-5 M respectively.
7 Ouabain partially inhibited, in a dose-dependent manner, Na+, K+-Mg2+ ATPase activity of the semi-purified plasma membranes.
8 The results demonstrate a good correlation between the ability of different drugs, known to inhibit ATPase activity, to displace [3H]-ouabain binding to adreno—medullary plasma membranes and their capacity to evoke a CA secretory response from isolated chromaffin cells. The data also suggest that the CA secretory effects of ouabain may not be due simply to inhibition of the Na+ pump and the subsequent ionic redistribution across the plasma membrane; a second mechanism may also be involved.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- Akera T., Brody T. M. The role of Na+,K+-ATPase in the inotropic action of digitalis. Pharmacol Rev. 1977 Sep;29(3):187–220. [PubMed] [Google Scholar]
- Akera T. Membrane adenosinetriphosphatase: a digitalis receptor? Science. 1977 Nov 11;198(4317):569–574. doi: 10.1126/science.144320. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. A note of the mechanism by which inhibitors of the sodium pump accelerate spontaneous release of transmitter from motor nerve terminals. J Physiol. 1975 May;247(1):209–226. doi: 10.1113/jphysiol.1975.sp010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks P. The effect of ouabain on the secretion of catecholamines and on the intracellular concentration of potassium. J Physiol. 1967 Dec;193(3):631–637. doi: 10.1113/jphysiol.1967.sp008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Josephson L., Warner R., Yanagisawa M., Lechene C., Guidotti G. Vanadate is a potent (Na,K)-ATPase inhibitor found in ATP derived from muscle. J Biol Chem. 1977 Nov 10;252(21):7421–7423. [PubMed] [Google Scholar]
- Dixon W. R., Garcia A. G., Kirpekar S. M. Release of catecholamines and dopamine beta-hydroxylase from the perfused adrenal gland of the cat. J Physiol. 1975 Jan;244(3):805–824. doi: 10.1113/jphysiol.1975.sp010827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. J. The action of ouabain in promoting the release of catecholamines. Experientia. 1977 Jul 15;33(7):923–924. doi: 10.1007/BF01951283. [DOI] [PubMed] [Google Scholar]
- Erdmann E., Krawietz W., Phillipp G., Hackbarth I., Schmitz W., Scholz H. Stimulatory effect of vanadate on (Na+ + K+)-ATPase activity and on 3H-ouabain-binding in a cat heart cell membrane preparation. Nature. 1979 Mar 29;278(5703):459–461. doi: 10.1038/278459a0. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Fajdiga P. B., Howe N. B., Livett B. G. Functional and morphological characterization of isolated bovine adrenal medullary cells. J Cell Biol. 1978 Jan;76(1):12–30. doi: 10.1083/jcb.76.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A. G., Kirpekar S. M. Letter: Inhibition of Na, K-activated ATPase and release of neurotransmitters. Nature. 1975 Oct 23;257(5528):722–722. doi: 10.1038/257722b0. [DOI] [PubMed] [Google Scholar]
- Garcia A. G., Kirpekar S. M. On the mechanism of release of norepinephrine from cat spleen slices by sodium deprivation and calcium pretreatment. J Pharmacol Exp Ther. 1975 Feb;192(2):343–350. [PubMed] [Google Scholar]
- Garcia A. G., Kirpekar S. M. Release of noradrenaline from the cat spleen by sodium deprivation. Br J Pharmacol. 1973 Apr;47(4):729–747. doi: 10.1111/j.1476-5381.1973.tb08200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysel-Burton J., Godfraind T. Stimulation and inhibition of the sodium pump by cardioactive steroids in relation to their binding sites and their inotropic effect on guinea-pig isolated atria. Br J Pharmacol. 1979 Jun;66(2):175–184. doi: 10.1111/j.1476-5381.1979.tb13662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hexum T. D. Studies on the reaction catalyzed by transport (Na, K) adenosine triphosphatase-II. In vitro and in vivo effects of phenoxybenzamine. Biochem Pharmacol. 1978;27(17):2109–2114. doi: 10.1016/0006-2952(78)90281-2. [DOI] [PubMed] [Google Scholar]
- Kirpekar S. M., Prat J. C., Yamamoto H. Effects of metabolic inhibitors on norepinephrine release from the perfused spleen of the cat. J Pharmacol Exp Ther. 1970 Apr;172(2):342–350. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaBella F. S., Bihler I., Kim R. S. Progesterone derivative binds to cardiac ouabain receptor and shows dissociation between sodium pump inhibition and increased contractile force. Nature. 1979 Apr 5;278(5704):571–573. doi: 10.1038/278571a0. [DOI] [PubMed] [Google Scholar]
- Lindmar R., Löffelholz K. The neuronal efflux of noradrenaline: dependency on sodium and facilitation by ouabain. Naunyn Schmiedebergs Arch Pharmacol. 1974;284(1):93–100. doi: 10.1007/BF00499974. [DOI] [PubMed] [Google Scholar]
- Nakazato Y., Ohga A., Onoda Y. The effect of ouabain on noradrenaline output from peripheral adrenergic neurones of isolated guinea-pig vas deferens. J Physiol. 1978 May;278:45–54. doi: 10.1113/jphysiol.1978.sp012291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor G. J., Dick D. A., Dick E. G., Le Poidevin D., Whyte S. F. Erythrocyte membrane cation carrier in depressive illness. Psychol Med. 1973 Nov;3(4):502–508. doi: 10.1017/s0033291700054313. [DOI] [PubMed] [Google Scholar]
- Vizi E. S. Na+-K+-activated adenosinetriphosphatase as a trigger in transmitter release. Neuroscience. 1978;3(4-5):367–384. doi: 10.1016/0306-4522(78)90040-4. [DOI] [PubMed] [Google Scholar]
- Wilson S. P., Kirshner N. Localization of adenylate cyclase in adrenal medulla. Mol Pharmacol. 1977 Mar;13(2):382–385. [PubMed] [Google Scholar]