Abstract
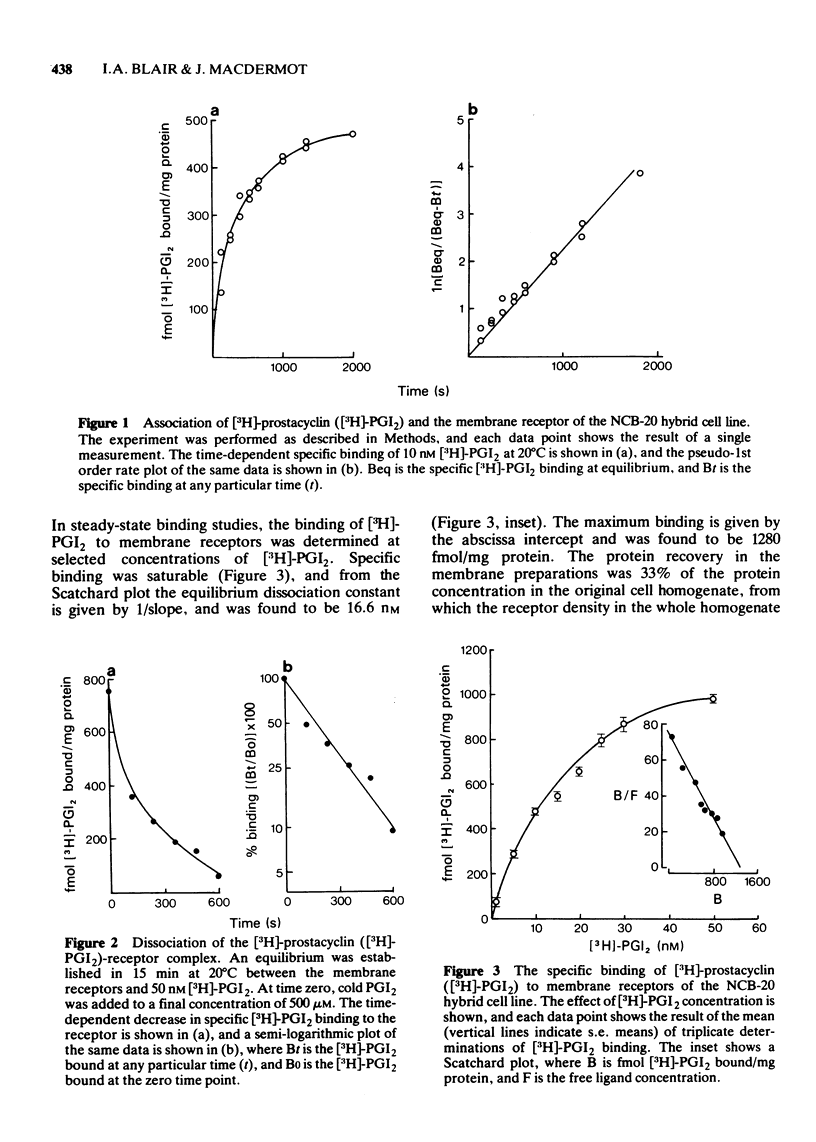
1 [3H]-prostacyclin bound to a washed membrane preparation of the NCB-20 neuronal hybrid cell line. 2 Kinetic analysis of [3H]-prostacyclin binding suggested a simple, non-cooperative bimolecular interaction between the ligand and a single receptor population. The equilibrium dissociation constant was 16.6 nM, and binding at a saturating [3H]-prostacyclin concentration enabled the receptor density of 2.57 x 10(5) receptor molecules per cell to be calculated. 3 At 20 degrees C the rate constant for the forward reaction (K+1) was 2.26 x 10(5) M-1 S-1, and the rate constant for the dissociation of the ligand-receptor complex (k-1) was 3.85 x 10(-3) S-1. Thus the dissociation constant (k-1/k+1) was 17.0 nM. 4 Prostaglandin E1 and prostacyclin compete for a single receptor in these cells, and comparison of other prostaglandins as inhibitors of [3H]-prostacyclin binding revealed some of the structural requirements for high-affinity occupation of prostacyclin receptors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair I. A., Hensby C. N., MacDermot J. Prostacyclin-dependent activation of adenylate cyclase in a neuronal somatic cell hybrid: prostanoid structure-activity relationships. Br J Pharmacol. 1980 Jul;69(3):519–525. doi: 10.1111/j.1476-5381.1980.tb07043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton L. L., Wiklund R. A., Van Arsdale P. M., Gilman A. G. Binding of (3H)prostaglandin E1 to putative receptors linked to adenylate cyclase of cultured cell clones. J Biol Chem. 1976 May 25;251(10):3037–3044. [PubMed] [Google Scholar]
- Hedqvist P. Actions of prostacyclin (PGI2) on adrenergic neuroeffector transmission in the rabbit kidney. Prostaglandins. 1979 Feb;17(2):249–258. doi: 10.1016/0090-6980(79)90045-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lad P. M., Welton A. F., Rodbell M. Evidence for distinct guanine nucleotide sites in the regulation of the glucagon receptor and of adenylate cyclase activity. J Biol Chem. 1977 Sep 10;252(17):5942–5946. [PubMed] [Google Scholar]
- Levitzki A. The role of GTP in the activation of adenylate cyclase. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1154–1159. doi: 10.1016/0006-291x(77)91639-4. [DOI] [PubMed] [Google Scholar]
- MacDermot J. Guanosine 5'-triphosphate reqiuirement for activation of adenylate cyclase by serotonin in a somatic cell hybrid. Life Sci. 1979 Jul 16;25(3):241–246. doi: 10.1016/0024-3205(79)90291-1. [DOI] [PubMed] [Google Scholar]
- MacDermot J., Higashida H., Wilson S. P., Matsuzawa H., Minna J., Nirenberg M. Adenylate cyclase and acetylcholine release regulated by separate serotonin receptors of somatic cell hybrids. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1135–1139. doi: 10.1073/pnas.76.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermot J., Nirenberg M. Turnover of opiate receptors in neuroblastoma X glioma hybrid cells. FEBS Lett. 1978 Jun 15;90(2):345–347. doi: 10.1016/0014-5793(78)80401-3. [DOI] [PubMed] [Google Scholar]
- Miller O. V., Gorman R. R. Evidence for distinct prostaglandin I2 and D2 receptors in human platelets. J Pharmacol Exp Ther. 1979 Jul;210(1):134–140. [PubMed] [Google Scholar]
- Minna J. D., Yavelow J., Coon H. G. Expression of phenotypes in hybrid somatic cells derived from the nervous system. Genetics. 1975 Jun;79 (Suppl):373–383. [PubMed] [Google Scholar]
- Minna J., Glazer D., Nirenberg M. Genetic dissection of neural properties using somatic cell hybrids. Nat New Biol. 1972 Feb 23;235(60):225–231. doi: 10.1038/newbio235225a0. [DOI] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Pert C. B., Snyder S. H. Properties of opiate-receptor binding in rat brain. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2243–2247. doi: 10.1073/pnas.70.8.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Sabol S. L., Nirenberg M. Regulation of adenylate cyclase of neuroblastoma x glioma hybrid cells by alpha-adrenergic receptors. I. Inhibition of adenylate cyclase mediated by alpha receptors. J Biol Chem. 1979 Mar 25;254(6):1913–1920. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Nirenberg M., Klee W. A. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975 Feb;72(2):590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl A. M., Smith J. B., Silver M. J., Nicolaou K. C., Ahern D. Selective binding site for [3H]prostacyclin on platelets. J Clin Invest. 1979 Feb;63(2):215–220. doi: 10.1172/JCI109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateson J. E., Moncada S., Vane J. R. Effects of prostacyclin (PGX) on cyclic AMP concentrations in human platelets. Prostaglandins. 1977 Mar;13(3):389–397. doi: 10.1016/0090-6980(77)90019-3. [DOI] [PubMed] [Google Scholar]
- Whittaker N., Bunting S., Salmon J., Moncada S., Vane J. R., Johnson R. A., Morton D. R., Kinner J. H., Gorman R. R., McGuire J. C. The chemical structure of prostaglandin X (prostacyclin). Prostaglandins. 1976 Dec;12(6):915–928. doi: 10.1016/0090-6980(76)90126-x. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Boughton-Smith N. K., Moncada S., Vane J. R. The relative activity of prostacyclin (PGI2) and a stable analogue 6beta-PGI1 on the gastrointestinal and cardiovascular systems. J Pharm Pharmacol. 1978 Sep;30(9):597–599. doi: 10.1111/j.2042-7158.1978.tb13338.x. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Moncada S., Vane J. R. Comparison of the effects of prostacyclin (PGI2), prostaglandin E1 and D2 on platelet aggregation in different species. Prostaglandins. 1978 Sep;16(3):373–388. doi: 10.1016/0090-6980(78)90216-2. [DOI] [PubMed] [Google Scholar]


