Abstract
1 The emetic action of Met-enkephalin, morphine and naloxone was studied following their administration into the cerebral ventricles of dogs through chronically implanted cannulae and the effect on the responses of ablating the chemorceptor trigger zone (CTZ) was investigated. The opiate antagonist, naloxone, was used to determine the role of enkephalin receptors in emetic responses.
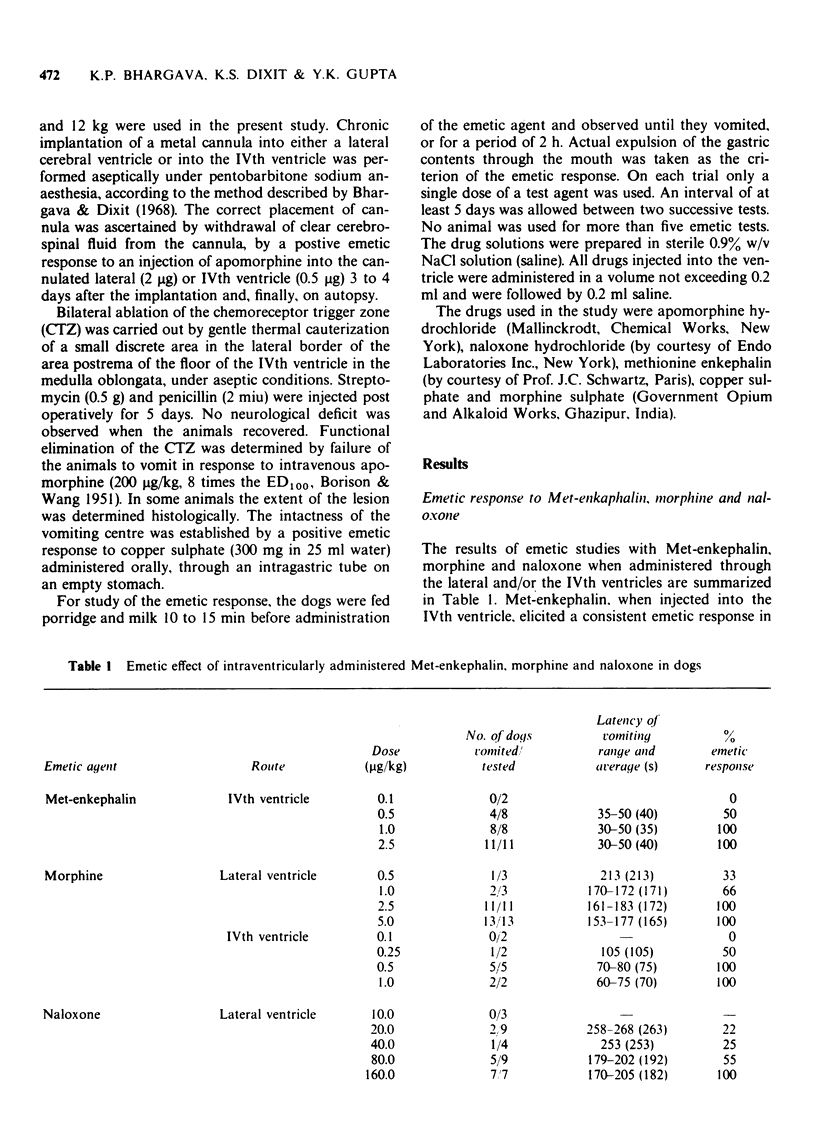
2 Administration of Met-enkephalin (1.0 μg/kg) into the IVth ventricle regularly evoked emesis with an average latency of 35 s. A dose of morphine (2.5 μg/kg) which was five times larger was required for a consistent emetic response when introduced into the lateral cerebral ventricle (i.c.v.) as compared to the dose required by the IVth ventricular route. The latency of emetic responses by the latter route of injection of morphine was shorter. This is in accord with an action of morphine on the emetic CTZ.
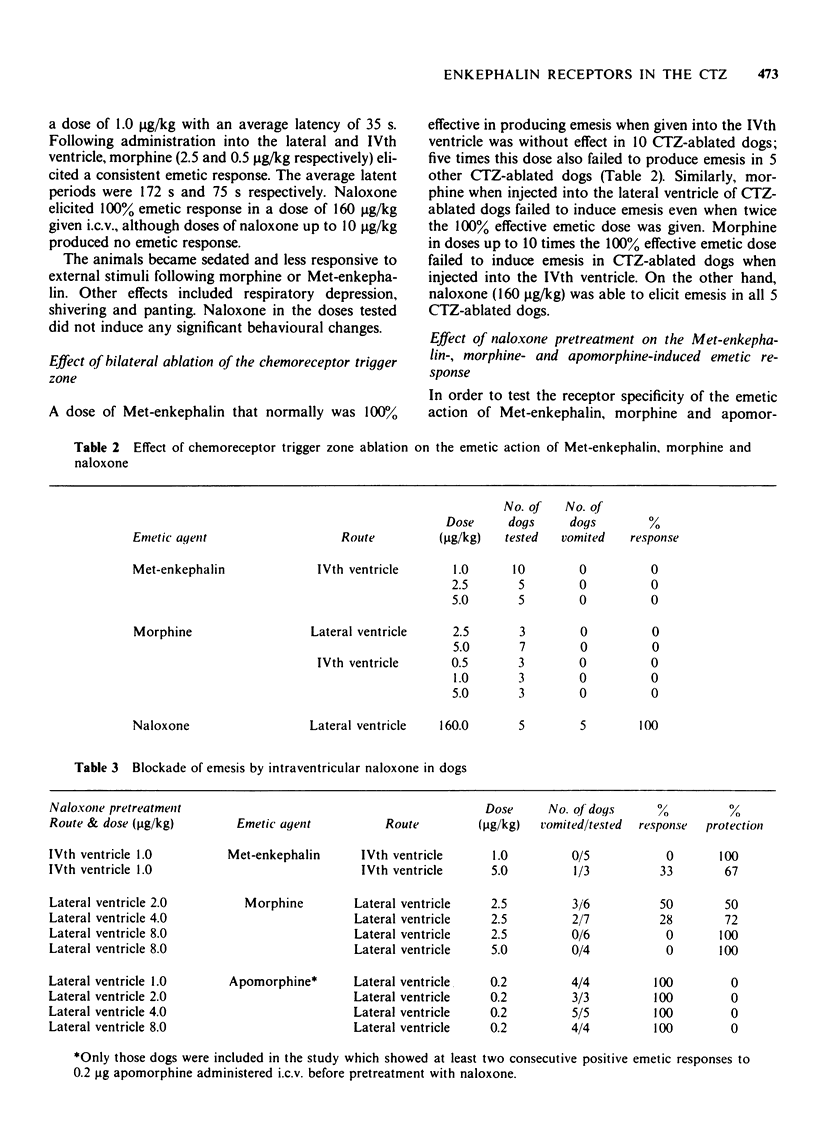
3 After bilateral ablation of the CTZ, intraventricular injections of Met-enkephalin and morphine failed to produce emesis even when given in doses that were 5 to 10 times the dose which regularly elicited emesis in animals with intact CTZ. The emesis produced in dogs by intraventricular Met-enkephalin and morphine is thus fully accounted for by an action on the CTZ.
4 Naloxone (i.c.v.) in doses up to 10.0 μg/kg did not cause emesis. However, higher doses of naloxone elicited dose-dependent emesis in dogs. The 100% emetic dose of naloxone was found to be 160 μg/kg and the latency of emesis was 180 s. Unlike Met-enkephalin and morphine, naloxone continued to elicit emesis in CTZ-ablated animals.
5 Pretreatment with intraventricular naloxone (1 to 8 μg/kg) blocked the emetic responses induced by intraventricular Met-enkephalin and morphine but not that to apomorphine. The selective protective action of the opiate antagonist against Met-enkephalin and morphine supports the presence of enkephalin receptors in the emetic CTZ.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atweh S. F., Kuhar M. J. Autoradiographic localization of opiate receptors in rat brain. II. The brain stem. Brain Res. 1977 Jun 24;129(1):1–12. doi: 10.1016/0006-8993(77)90965-9. [DOI] [PubMed] [Google Scholar]
- BHARGAVA K. P., GUPTA P. C., CHANDRA O. M. Effect of ablation of the chemoreceptor trigger zone (CT zone) on the emetic response to intraventricular injection of apomorphine and emetine in the dog. J Pharmacol Exp Ther. 1961 Dec;134:329–331. [PubMed] [Google Scholar]
- BORISON H. L., FISHBURN B. R., BHIDE N. K., McCARTHY L. E. Morphine-induced hyperglycemia in the cat. J Pharmacol Exp Ther. 1962 Nov;138:229–235. [PubMed] [Google Scholar]
- BORISON H. L., WANG S. C. Locus of the central emetic action of cardiac glycosides. Proc Soc Exp Biol Med. 1951 Feb;76(2):335–338. doi: 10.3181/00379727-76-18482. [DOI] [PubMed] [Google Scholar]
- Bhargava K. P., Dixit K. S., Palit G. Nature of histamine receptors in the emetic chemoreceptor trigger zone. Br J Pharmacol. 1976 Jun;57(2):211–213. doi: 10.1111/j.1476-5381.1976.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava K. P., Dixit K. S. Role of the chemoreceptor trigger zone in histamine-induced emesis. Br J Pharmacol. 1968 Nov;34(3):508–513. doi: 10.1111/j.1476-5381.1968.tb08479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin D. H., George R., Li C. H. Beta-endorphin: pharmacologic and behavioral activity in cats after low intravenous doses. Life Sci. 1978 Nov 20;23(21):2147–2154. doi: 10.1016/0024-3205(78)90188-1. [DOI] [PubMed] [Google Scholar]
- Costello D. J., Borison H. L. Naloxone antagonizes narcotic self blockade of emesis in the cat. J Pharmacol Exp Ther. 1977 Oct;203(1):222–230. [PubMed] [Google Scholar]
- Hughes J. Isolation of an endogenous compound from the brain with pharmacological properties similar to morphine. Brain Res. 1975 May 2;88(2):295–308. doi: 10.1016/0006-8993(75)90391-1. [DOI] [PubMed] [Google Scholar]
- Johansson O., Hökfelt T., Elde R. P., Schultzberg M., Terenius L. Immunohistochemical distribution of enkephalin neurons. Adv Biochem Psychopharmacol. 1978;18:51–70. [PubMed] [Google Scholar]
- Kosterlitz H. W., Leslie F. M. Comparison of the receptor binding characteristics of opiate agonists interacting with mu- or kappa-receptors. Br J Pharmacol. 1978 Dec;64(4):607–614. doi: 10.1111/j.1476-5381.1978.tb17323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simantov R., Snyder S. H. Morphine-like peptides in mammalian brain: isolation, structure elucidation, and interactions with the opiate receptor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2515–2519. doi: 10.1073/pnas.73.7.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TPERENIUS L., Wahlström A. Morphine-like ligand for opiate receptors in human CSF. Life Sci. 1975 Jun 15;16(12):1759–1764. doi: 10.1016/0024-3205(75)90269-6. [DOI] [PubMed] [Google Scholar]
- WANG S. C., GLAVIANO V. V. Locus of emetic action of morphine and hydergine in dogs. J Pharmacol Exp Ther. 1954 Jul;111(3):329–334. [PubMed] [Google Scholar]


