Abstract
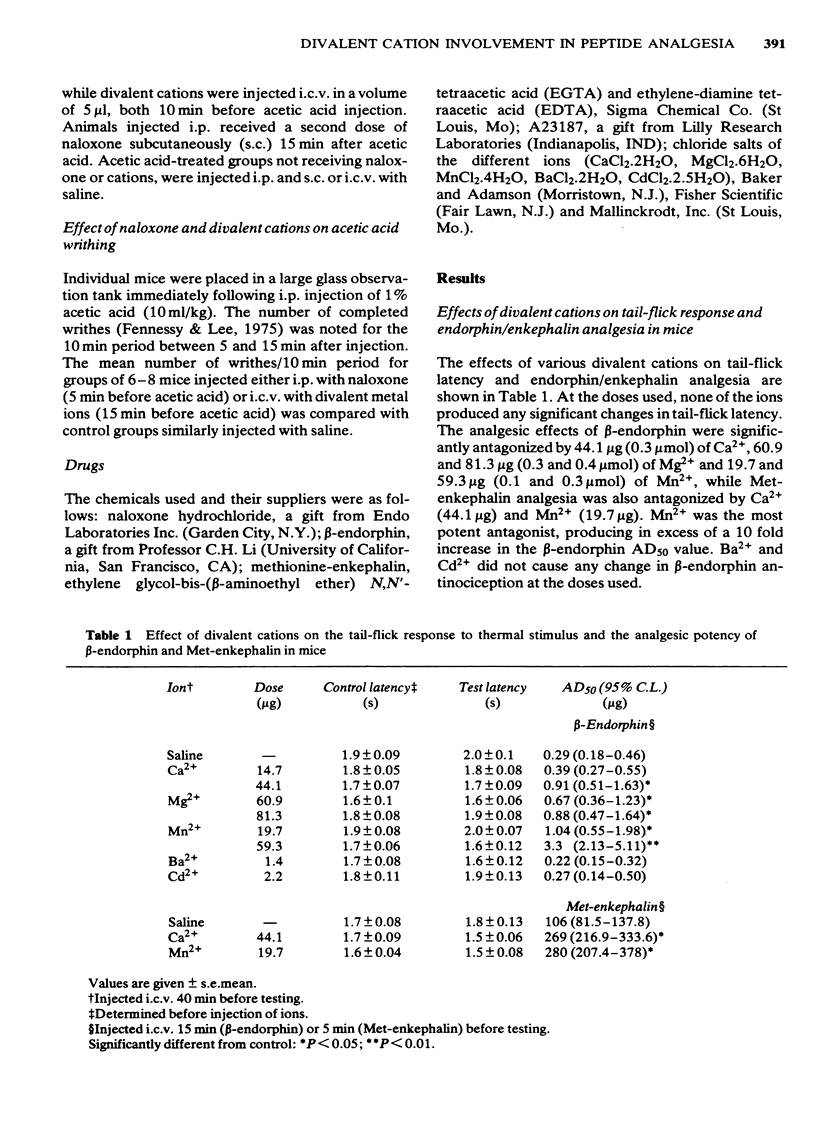
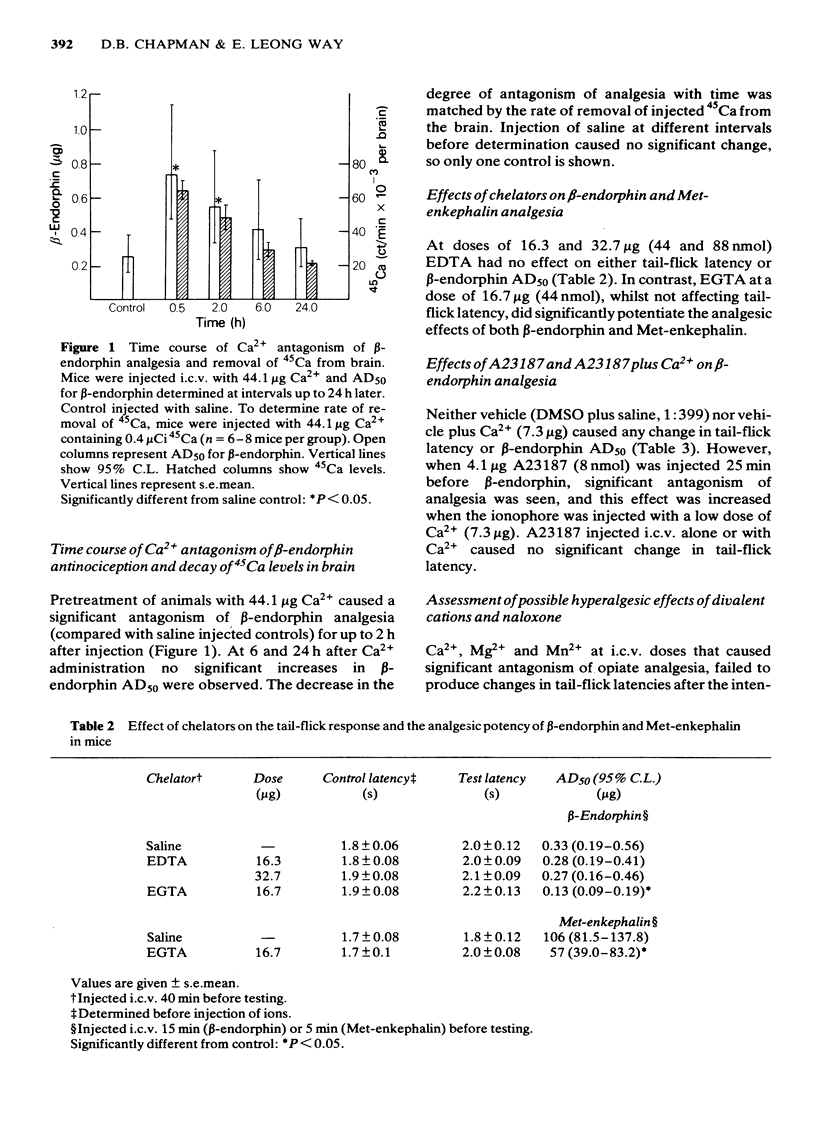
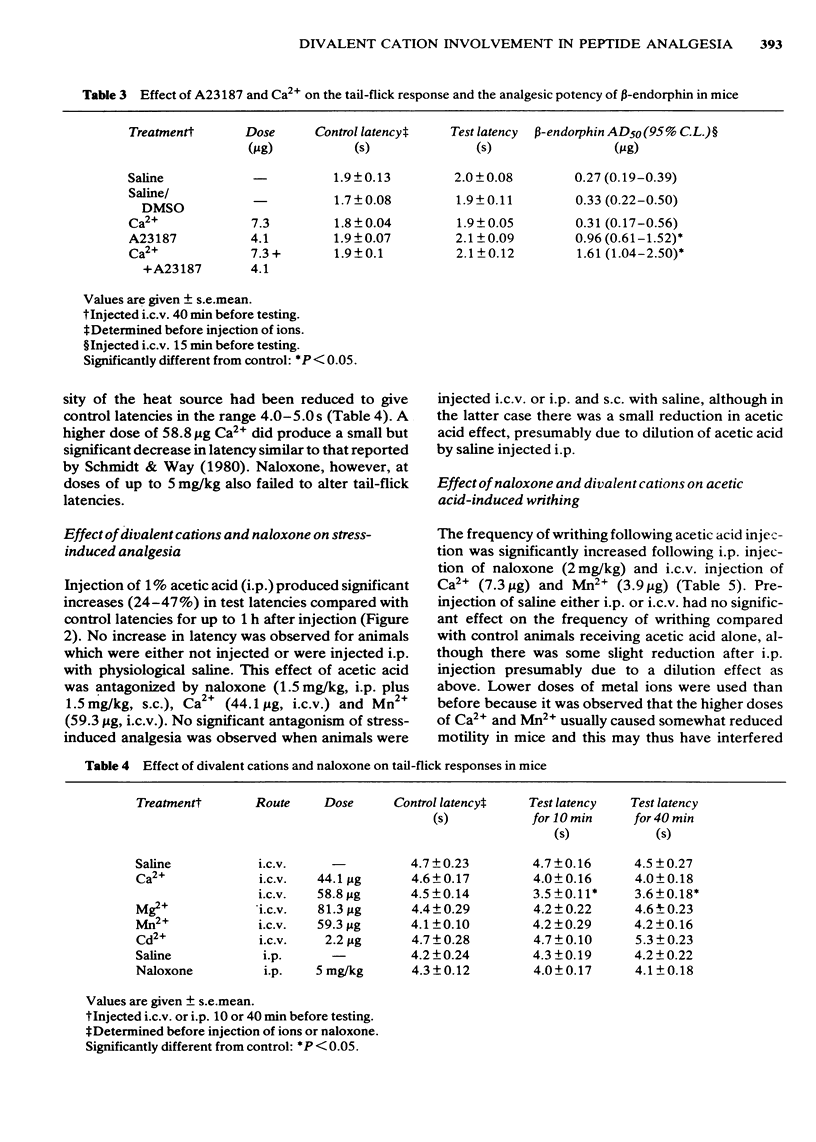
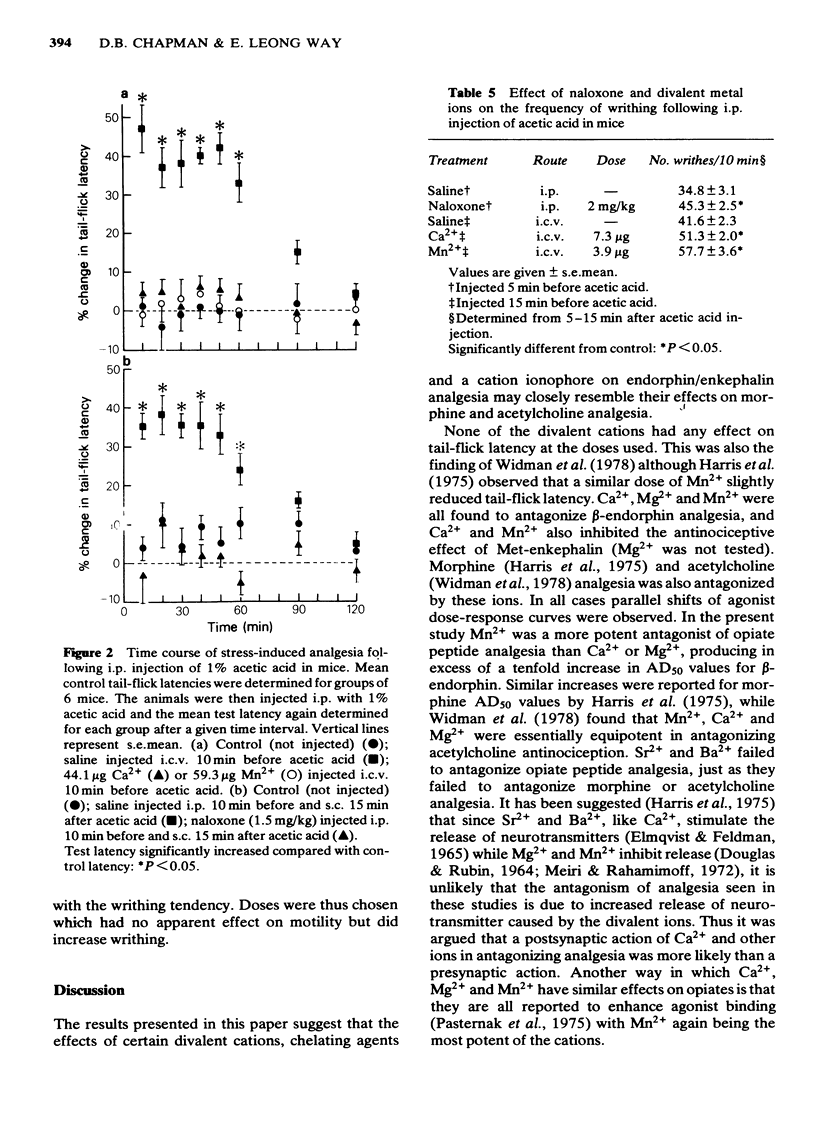
The possibility that divalent cations may antagonize opiate peptide analgesia and stress-induced analgesia was examined. Intracerebroventricular injection of low doses of Ca2+, Mn2+ and Mg2+ antagonized beta-endorphin and methionine-enkephalin analgesia. Ba2+ and Cd2+ were without effect. The ionophore, A23187, significantly antagonized beta-endorphin analgesia and the effect was increased when a low dose of Ca2+ was injected at the same time as the ionophore. Ethylene glycol tetraacetic acid (but not ethylenediamine tetraacetic acid) significantly potentiated endorphin analgesia. Stress-induced analgesia, as determined by increased tail-flick latencies following intraperitoneal injection of acetic acid, was effectively antagonized by naloxone, Ca2+ and Mn2+. The frequency of writhing following acetic acid injection was increased by both naloxone and divalent metal ions, again suggesting antagonism of endogenous opiates. These results confirm previous findings indicating that divalent metal ions (and especially Ca2+) may be involved in the actions of opiates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belluzzi J. D., Grant N., Garsky V., Sarantakis D., Wise C. D., Stein L. Analgesia induced in vivo by central administration of enkephalin in rat. Nature. 1976 Apr 15;260(5552):625–626. doi: 10.1038/260625a0. [DOI] [PubMed] [Google Scholar]
- Chapman D. B., Way E. L. Metal ion interactions with opiates. Annu Rev Pharmacol Toxicol. 1980;20:553–579. doi: 10.1146/annurev.pa.20.040180.003005. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. THE EFFECTS OF ALKALINE EARTHS AND OTHER DIVALENT CATIONS ON ADRENAL MEDULLARY SECRETION. J Physiol. 1964 Dec;175:231–241. doi: 10.1113/jphysiol.1964.sp007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT H. W., KOKKA N., WAY E. L. INFLUENCE OF CALCIUM-DEFICIT ON MORPHINE INHIBITION OF QO2 OF RAT CEREBRAL CORTEX SLICES. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:1049–1052. doi: 10.3181/00379727-113-28569. [DOI] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Calcium dependence of spontaneous acetylcholine release at mammalian motor nerve terminals. J Physiol. 1965 Dec;181(3):487–497. doi: 10.1113/jphysiol.1965.sp007777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Munoz F., Cerreta K. V., Guerrero M. L., Way E. L. Effect of morphine on synaptosomal Ca++ uptake. J Pharmacol Exp Ther. 1979 Apr;209(1):132–136. [PubMed] [Google Scholar]
- Göthert M., Pohl I. M., Wehking E. Effects of presynaptic modulators on Ca2+-induced noradrenaline release from central noradrenergic neurons. Noradrenaline and enkephalin inhibit release by decreasing depolarization-induced Ca2+ influx. Naunyn Schmiedebergs Arch Pharmacol. 1979 May;307(1):21–27. doi: 10.1007/BF00506547. [DOI] [PubMed] [Google Scholar]
- HANO K., KANETO H., KAKUNAGA T. SIGNIFICANCE OF CALCIUM ION IN THE MORPHINE ANALGESIA. Jpn J Pharmacol. 1964 Jun;14:227–229. doi: 10.1254/jjp.14.227. [DOI] [PubMed] [Google Scholar]
- Harris R. A., Loh H. H., Way E. L. Effects of divalent cations, cation chelators and an ionophore on morphine analgesia and tolerance. J Pharmacol Exp Ther. 1975 Dec;195(3):488–498. [PubMed] [Google Scholar]
- Harris R. A., Yamamoto H., Loh H. H., Way E. L. Discrete changes in brain calcium with morphine analgesia, tolerance-dependence, and abstinence. Life Sci. 1977 Feb 1;20(3):501–505. doi: 10.1016/0024-3205(77)90393-9. [DOI] [PubMed] [Google Scholar]
- Kakunaga T., Kaneto H., Hano K. Pharmacologic studies on analgesics. VII. Significance of the calcium ion in morphine analgesia. J Pharmacol Exp Ther. 1966 Jul;153(1):134–141. [PubMed] [Google Scholar]
- Kakunaga T. [Pharmacological studies on analgesics. (IX). Significance of calcium in the mechanism of morphine analgesia. (3)]. Nihon Yakurigaku Zasshi. 1966 Mar 20;62(2):40–50. [PubMed] [Google Scholar]
- Kokka N., Fairhurst A. S. Naloxone enhancement of acetic acid-induced writhing in rats. Life Sci. 1977 Oct 1;21(7):975–980. doi: 10.1016/0024-3205(77)90264-8. [DOI] [PubMed] [Google Scholar]
- Loh H. H., Tseng L. F., Wei E., Li C. H. beta-endorphin is a potent analgesic agent. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2895–2898. doi: 10.1073/pnas.73.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri U., Rahamimoff R. Neuromuscular transmission: inhibition by manganese ions. Science. 1972 Apr 21;176(4032):308–309. doi: 10.1126/science.176.4032.308. [DOI] [PubMed] [Google Scholar]
- Pasternak G. W., Snowman A. M., Snyder S. H. Selective enhancement of [3H]opiate agonist binding by divalent cations. Mol Pharmacol. 1975 Nov;11(6):735–744. [PubMed] [Google Scholar]
- Pressman B. C. Properties of ionophores with broad range cation selectivity. Fed Proc. 1973 Jun;32(6):1698–1703. [PubMed] [Google Scholar]
- Robinson I. C., Russell J. T., Thorn N. A. Calcium and stimulus secretion coupling in the neurohypophysis. V. The effects of the Ca2+ ionophores A23187 and X537 A on vasopressin release and 45Ca2+ efflux; interactions with sodium and a verapamil analogue (D600). Acta Endocrinol (Copenh) 1976 Sep;83(1):36–49. [PubMed] [Google Scholar]
- Ross D. H., Lynn S. C., Jr, Cardenas H. L. Selective control of calcium levels by naloxone. Life Sci. 1976 Apr 15;18(8):789–795. doi: 10.1016/0024-3205(76)90003-5. [DOI] [PubMed] [Google Scholar]
- Schmidt W. K., Way E. L. Hyperalgesic effects of divalent cations and antinociceptive effects of a calcium chelator in naive and morphine-dependent mice. J Pharmacol Exp Ther. 1980 Jan;212(1):22–27. [PubMed] [Google Scholar]
- Widman M., Rosin D., Dewey W. L. Effects of divalent cations, lanthanum, cation chelators and an ionophore on acetylcholine antinociception. J Pharmacol Exp Ther. 1978 May;205(2):311–318. [PubMed] [Google Scholar]
- Yamamoto H., Harris R. A., Loh H. H., Way E. L. Effects of acute and chronic morphine treatments on calcium localization and binding in brain. J Pharmacol Exp Ther. 1978 May;205(2):255–264. [PubMed] [Google Scholar]


