Abstract
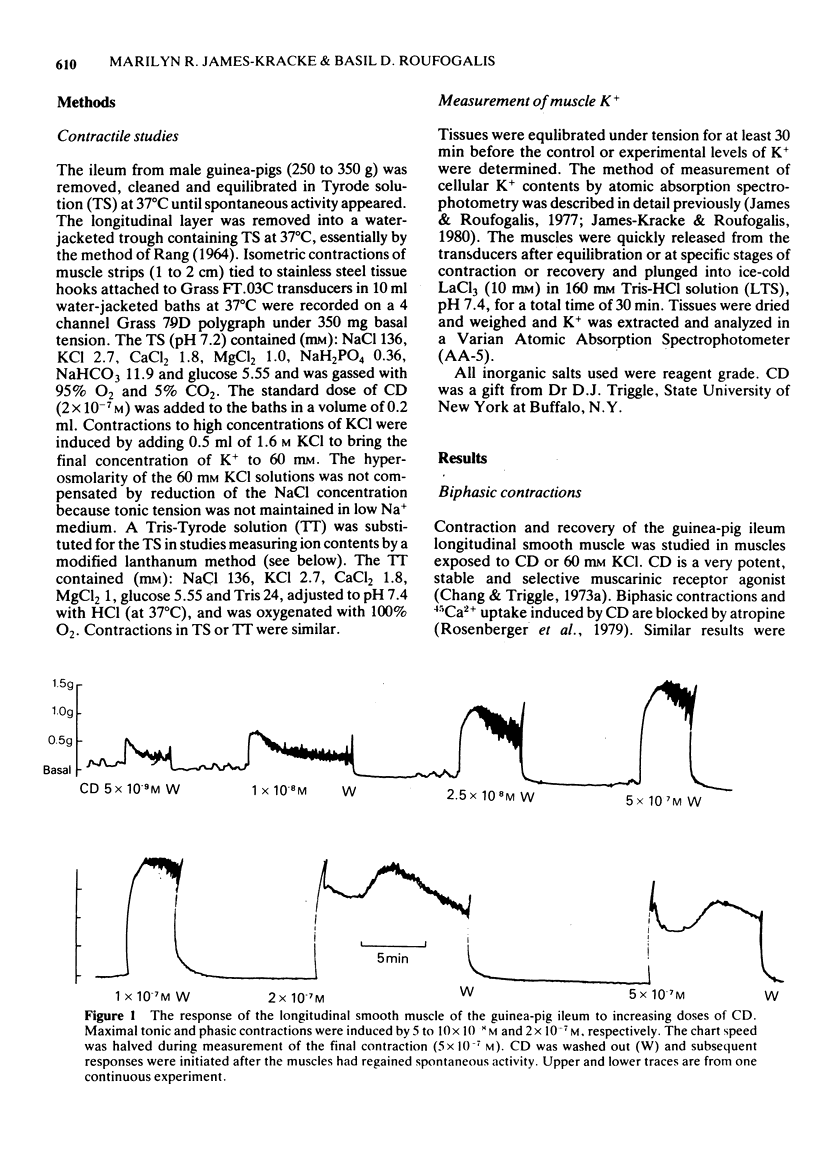
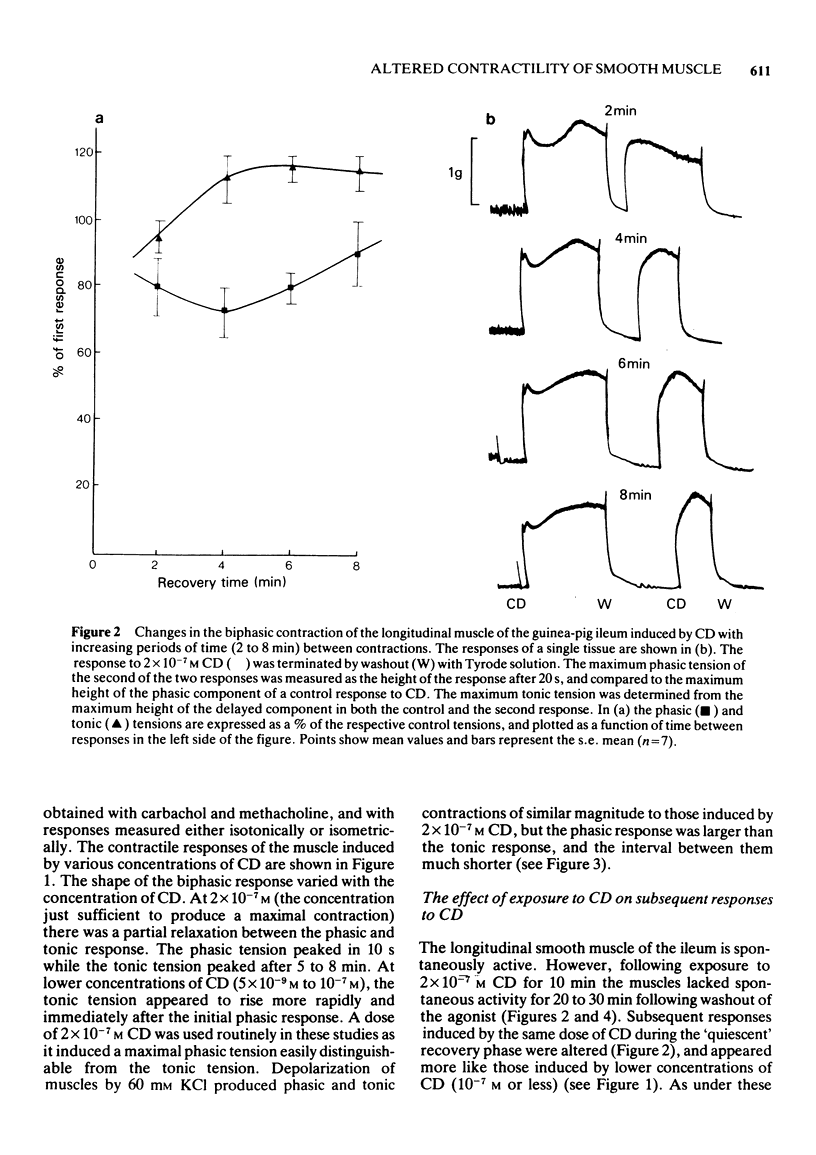
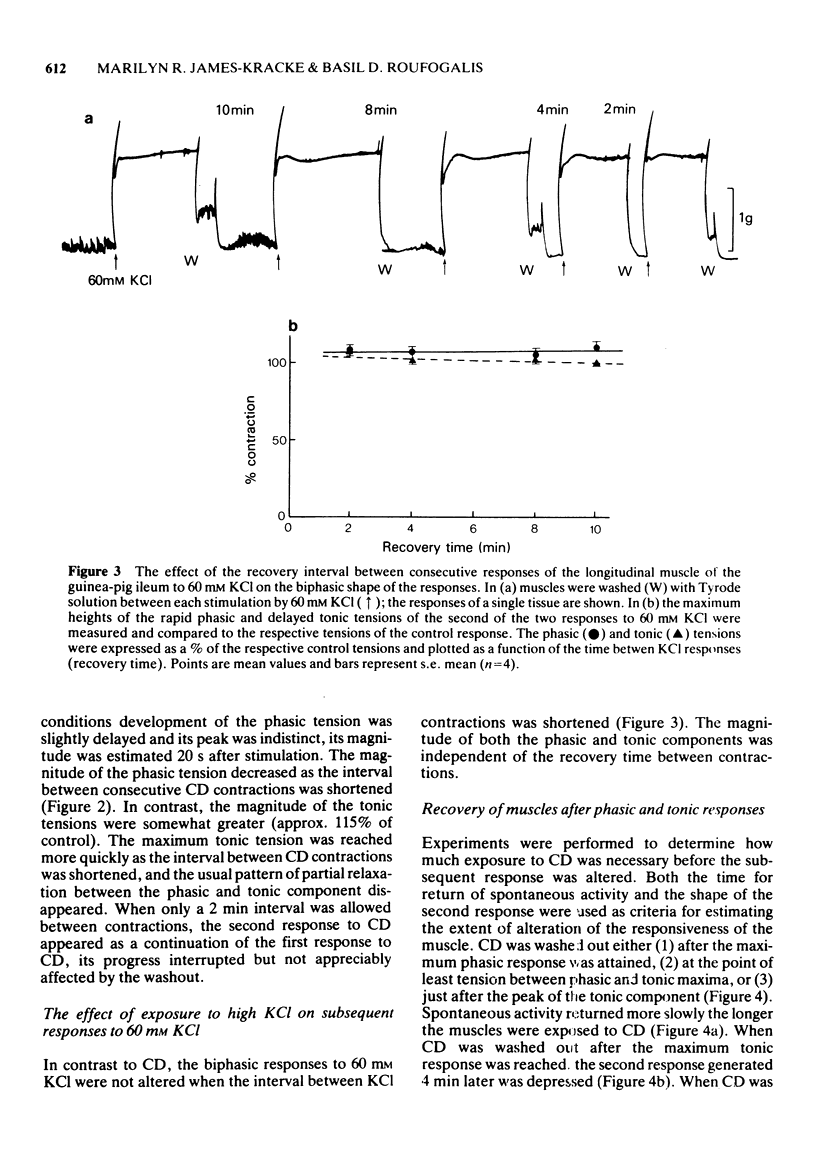
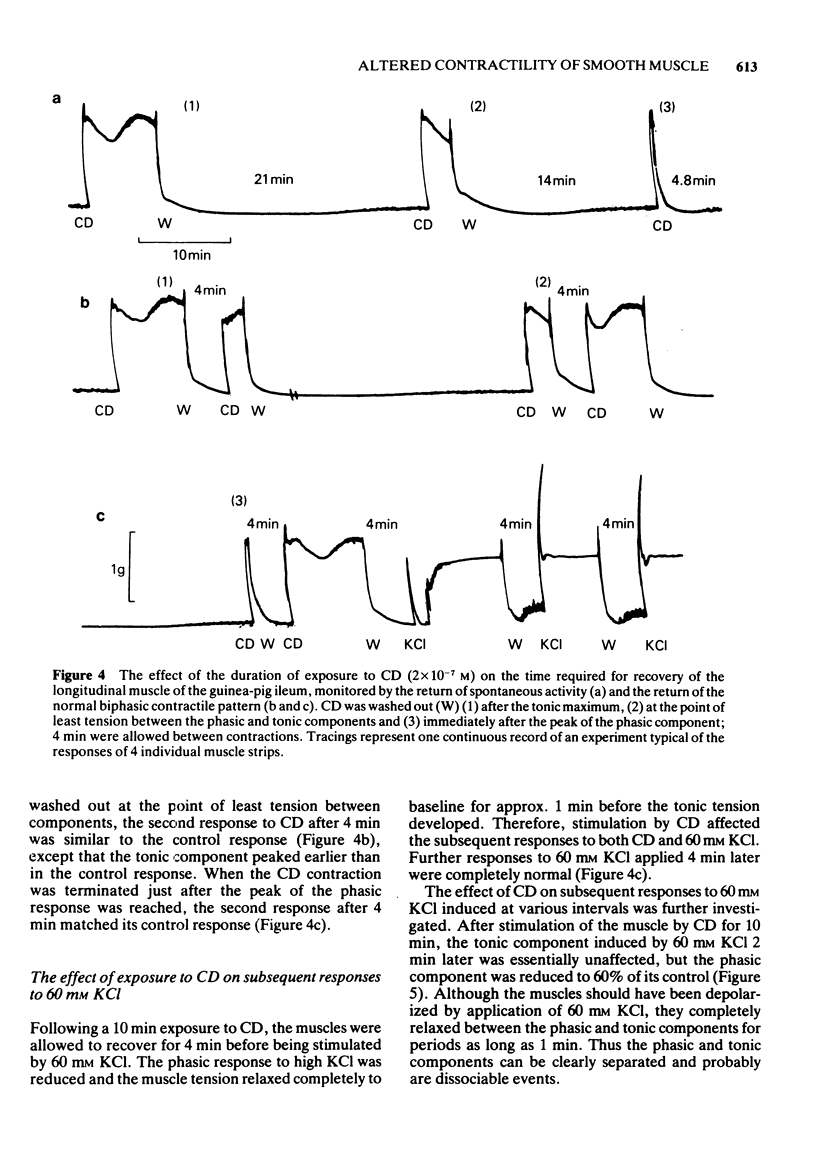
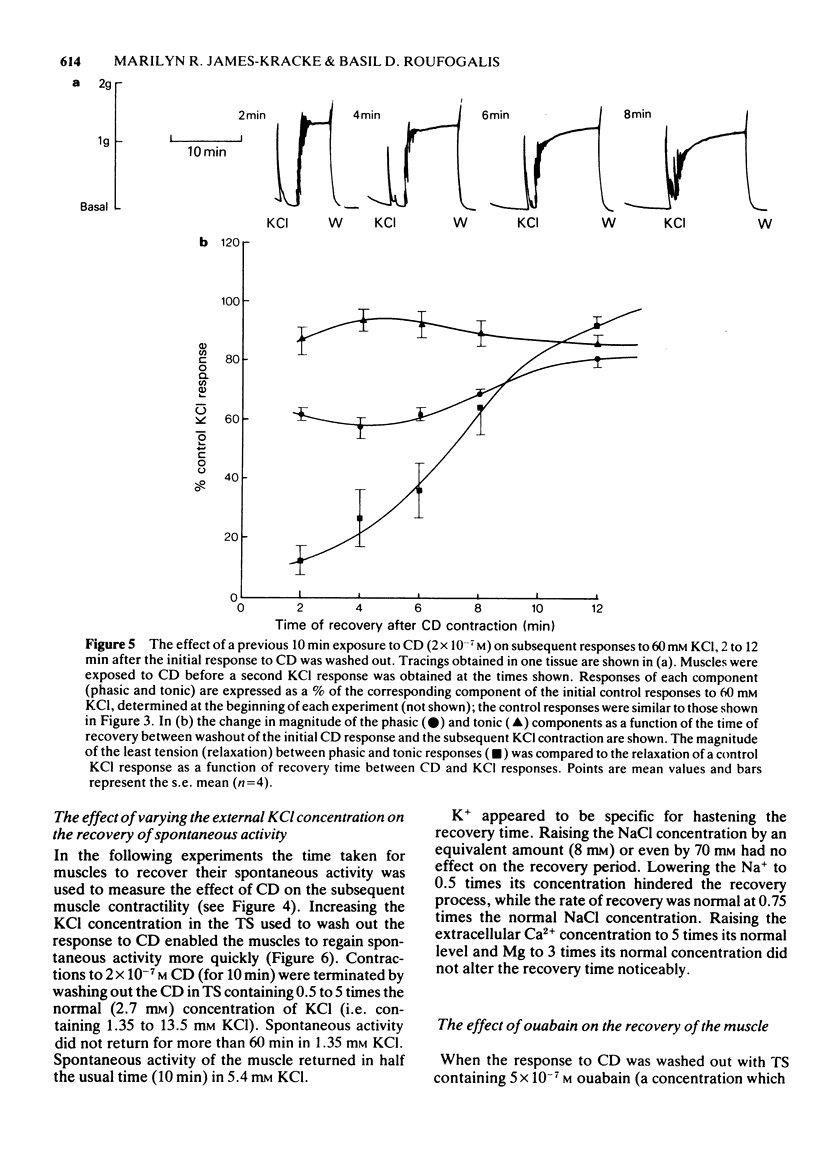
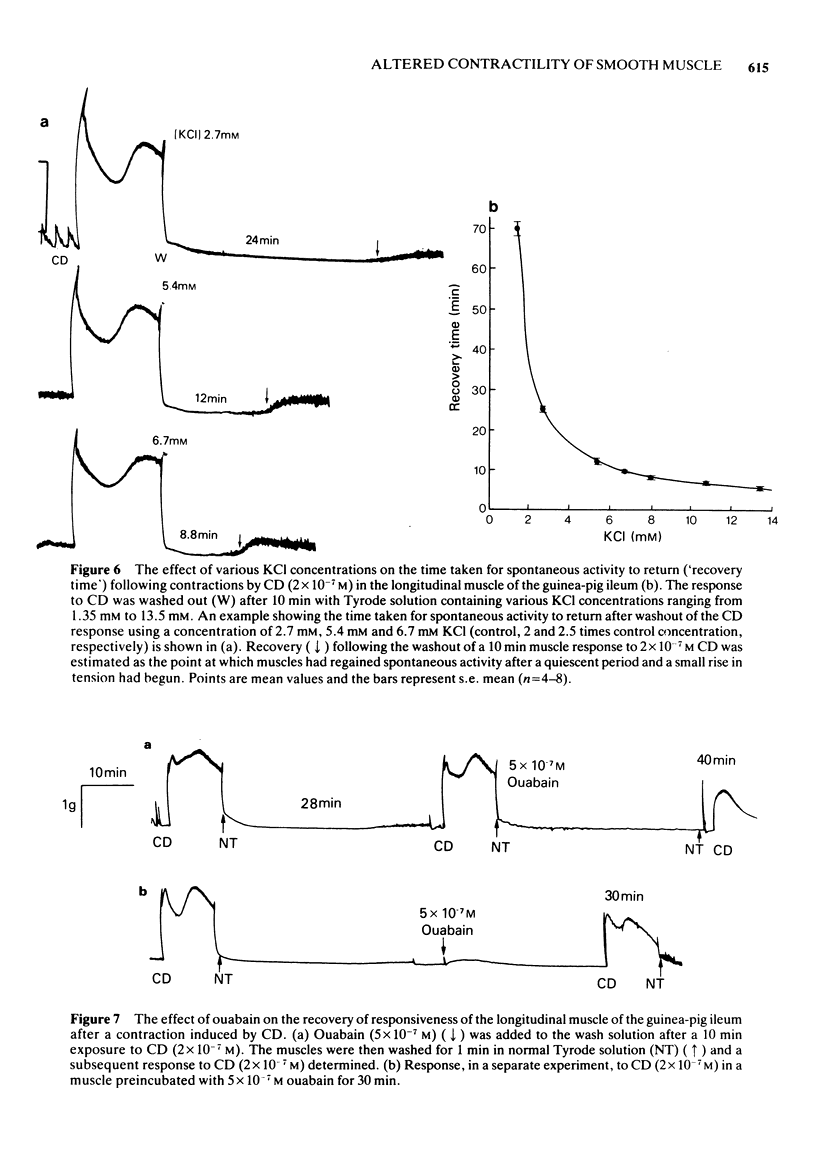
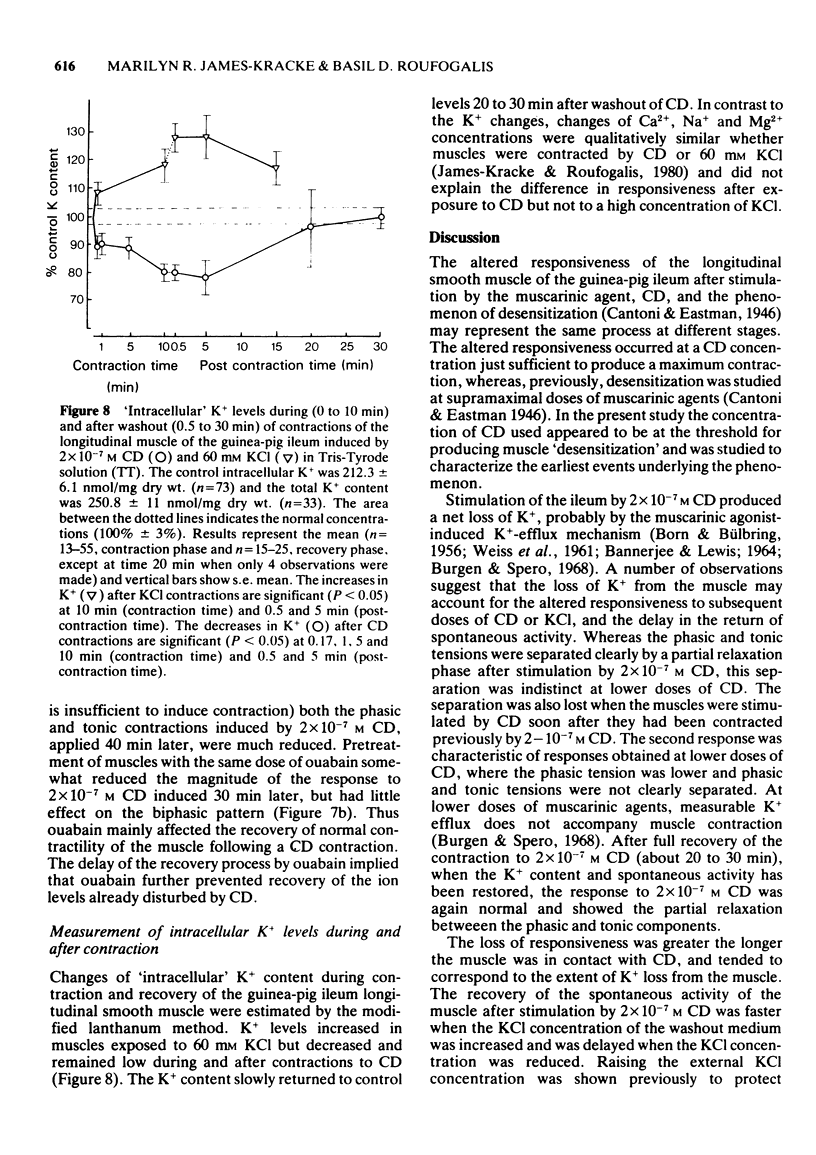
1. After stimulation of the longitudinal smooth muscle of the guinea-pig ileum by an optimal dose (2 x 10(-7) M) of a muscarinic agent, cis-2-methyl-4-dimethylaminomethyl-1,3-dioxolane methiodide (CD), the muscles failed to regain their normal spontaneous activity for 20 to 30 min. During the recovery period, subsequent contractions induced by either CD or 60 mM KCl were altered, particularly when only short times (15 min or less) were allowed between exposures. 2. Altered responses to CD had depressed phasic but increased tonic tensions and were characteristic of responses induced by lower doses of CD. The altered responsiveness probably represented an early phase of muscle 'densensitization'. 3. In contrast to muscarinic stimulation, the smooth muscles gave identical responses after repeated stimulation by 60 mM KCl, even when only 2 min were allowed between exposures. 4. Whereas K+ levels increased in muscles exposed to 60 mM KCl, they decreased during contractions to CD. The K+ levels remained low until the muscles recovered their normal responsiveness. 5. Increasing the extracellular K+ concentration (5 to 13 mM) hastened the recovery of the muscle responsiveness after CD, whereas lowering external K+ concentration to 1.35 mM or the addition of ouabain (5 x 10(-7) M) delayed the recovery. The results suggested that the Na+, K+-pump is rate-limiting in the recovery of the normal ionic balance of the muscles after stimulation by muscarinic agonists.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANERJEE A. K., LEWIS J. J. EFFECTS OF SMOOTH MUSCLE STIMULANTS AND THEIR ANATAGONISTS UPON POTASSIUM ION UPTAKE AND RELEASE IN STRIPS OF GUINEA-PIG ILEUM. J Pharm Pharmacol. 1964 Feb;16:134–136. doi: 10.1111/j.2042-7158.1964.tb07436.x. [DOI] [PubMed] [Google Scholar]
- BORN G. V., BULBRING E. The movement of potassium between smooth muscle and the surrounding fluid. J Physiol. 1956 Mar 28;131(3):690–703. doi: 10.1113/jphysiol.1956.sp005494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., STRAUB R. W. A method for studying the effects of ions and drugs on the resting and action potentials in smooth muscle with external electrodes. J Physiol. 1958 Jan 23;140(1):156–167. doi: 10.1113/jphysiol.1958.sp005924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsall N. J., Burgen A. S., Hulme E. C. The binding of agonists to brain muscarinic receptors. Mol Pharmacol. 1978 Sep;14(5):723–736. [PubMed] [Google Scholar]
- Blond D. M., Whittam R. Effects of sodium and potassium ions on oxidative phosphorylation in relation to respiratory control by a cell-membrane adenosine triphosphatase. Biochem J. 1965 Nov;97(2):523–531. doi: 10.1042/bj0970523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. The role of electrogenic sodium pumping in the response of smooth muscle to acetylcholine. J Physiol. 1973 Feb;228(3):713–731. doi: 10.1113/jphysiol.1973.sp010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F. Calcium-induced increase in membrane permeability in the guinea-pig taenia coli: evidence for involvement of a sodium-calcium exchange mechanism. J Physiol. 1978 Feb;275:65–84. doi: 10.1113/jphysiol.1978.sp012178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgen A. S., Spero L. The action of acetylcholine and other drugs on the efflux of potassium and rubidium from smooth muscle of the guinea-pig intestine. Br J Pharmacol. 1968 Sep;34(1):99–115. doi: 10.1111/j.1476-5381.1968.tb07954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygrave F. L. The ionic environment and metabolic control. Nature. 1967 May 13;214(5089):667–671. doi: 10.1038/214667a0. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Burnstock G. Membrane potential changes associated with tachyphylaxis and potentiation of the response to stimulating drugs in smooth muscle. Br J Pharmacol Chemother. 1960 Dec;15(4):611–624. doi: 10.1111/j.1476-5381.1960.tb00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Active ion transport and resting potential in smooth muscle cells. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):47–56. doi: 10.1098/rstb.1973.0008. [DOI] [PubMed] [Google Scholar]
- Chang K. J., Triggle D. J. Quantitative aspects of drug-receptor interactions. I. Ca2+ and cholinergic receptor activation in smooth muscle: a basic model for drug-receptor interactions. J Theor Biol. 1973 Jul;40(1):125–154. doi: 10.1016/0022-5193(73)90168-9. [DOI] [PubMed] [Google Scholar]
- Chang K. J., Triggle D. J. Quantitative aspects of drug-receptor interactions. II. The role of Ca2+men in desensitization and spasmolytic activity. J Theor Biol. 1973 Jul;40(1):155–172. doi: 10.1016/0022-5193(73)90169-0. [DOI] [PubMed] [Google Scholar]
- DURBIN R. P., JENKINSON D. H. The effect of carbachol on the permeability of depolarized smooth muscle to inorganic ions. J Physiol. 1961 Jun;157:74–89. doi: 10.1113/jphysiol.1961.sp006706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E. E., Janis R. A. Calcium regulation in the uterus. Pharmacol Ther B. 1975;1(4):695–729. doi: 10.1016/0306-039x(75)90025-2. [DOI] [PubMed] [Google Scholar]
- HURWITZ L. Potassium transport in isolated guinea pig ileum. Am J Physiol. 1960 Jan;198:94–98. doi: 10.1152/ajplegacy.1960.198.1.94. [DOI] [PubMed] [Google Scholar]
- HURWITZ L., TINSLEY B., BATTLE F. Dissociation of contraction and potassium efflux in smooth muscle. Am J Physiol. 1960 Jul;199:107–111. doi: 10.1152/ajplegacy.1960.199.1.107. [DOI] [PubMed] [Google Scholar]
- Hurwitz L., Joiner P. D. Excitation-contraction coupling in smooth muscle. Fed Proc. 1969 Sep-Oct;28(5):1629–1633. [PubMed] [Google Scholar]
- James M. R., Roufogalis B. D. The effect of ouabain on the guinea pig ileum longitudinal smooth muscle: 2. Intracellular levels of Ca, Na, K, and Mg during the ouabain response and the dependence of the response on extracellular Ca. Can J Physiol Pharmacol. 1977 Oct;55(5):1197–1203. doi: 10.1139/y77-163. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keryer G., Rossignol B. Lanthanum as a tool to study the role of phosphatidylinositol in the calcium transport in rat parotid glands upon cholinergic stimulation. Eur J Biochem. 1978 Apr;85(1):77–83. doi: 10.1111/j.1432-1033.1978.tb12213.x. [DOI] [PubMed] [Google Scholar]
- Magaribuchi T., Ito Y., Kuriyama H. Desensitization of smooth muscle cells in the guinea pig taenia coli to prolonged application of carbachol. Jpn J Physiol. 1973 Oct;23(5):447–464. doi: 10.2170/jjphysiol.23.447. [DOI] [PubMed] [Google Scholar]
- Marshall J. M., Kroeger E. A. Adrenergic influences on uterine smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):135–148. doi: 10.1098/rstb.1973.0016. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Nastuk W. L., Parsons R. L. Factors in the inactivation of postjunctional membrane receptors of frog skeletal muscle. J Gen Physiol. 1970 Aug;56(2):218–249. doi: 10.1085/jgp.56.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON W. D., ROTHSCHILD A. M. THE CHANGES IN RESPONSE AND IN IONIC CONTENT OF SMOOTH MUSCLE PRODUCED BY ACETYLCHOLINE ACTON AND BY CALCIUM DEFICIENCY. Br J Pharmacol Chemother. 1965 Apr;24:437–448. doi: 10.1111/j.1476-5381.1965.tb01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND M. J. The effect of potassium and acetylcholine on the response of the guinea-pig jejunum to histamine. Aust J Exp Biol Med Sci. 1957 Feb;35(1):79–82. doi: 10.1038/icb.1957.9. [DOI] [PubMed] [Google Scholar]
- RANG H. P. STIMULANT ACTIONS OF VOLATILE ANAESTHETICS ON SMOOTH MUSCLE. Br J Pharmacol Chemother. 1964 Apr;22:356–365. doi: 10.1111/j.1476-5381.1964.tb02040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger L. B., Ticku M. K., Triggle D. J. The effect of Ca2+ antagonists on mechanical responses and Ca2+ movements in guinea pig ileal longitudinal smooth muscle. Can J Physiol Pharmacol. 1979 Apr;57(4):333–347. doi: 10.1139/y79-052. [DOI] [PubMed] [Google Scholar]
- Tomita T., Watanabe H. Factors controlling myogenic activity in smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):73–85. doi: 10.1098/rstb.1973.0010. [DOI] [PubMed] [Google Scholar]
- Triggle C. R., Triggle D. J. An analysis of the action of cations of the lanthanide series on the mechanical responses of guinea-pig ileal longitudinal muscle. J Physiol. 1976 Jan;254(1):39–54. doi: 10.1113/jphysiol.1976.sp011219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen C. Calcium requirement for activation of intact aortic smooth muscle. J Physiol. 1977 Nov;272(2):317–329. doi: 10.1113/jphysiol.1977.sp012046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS G. B., COALSON R. E., HURWITZ L. K transport and mechanical responses of isolated longitudinal smooth muscle from guinea pig ileum. Am J Physiol. 1961 Apr;200:789–793. doi: 10.1152/ajplegacy.1961.200.4.789. [DOI] [PubMed] [Google Scholar]


