Abstract
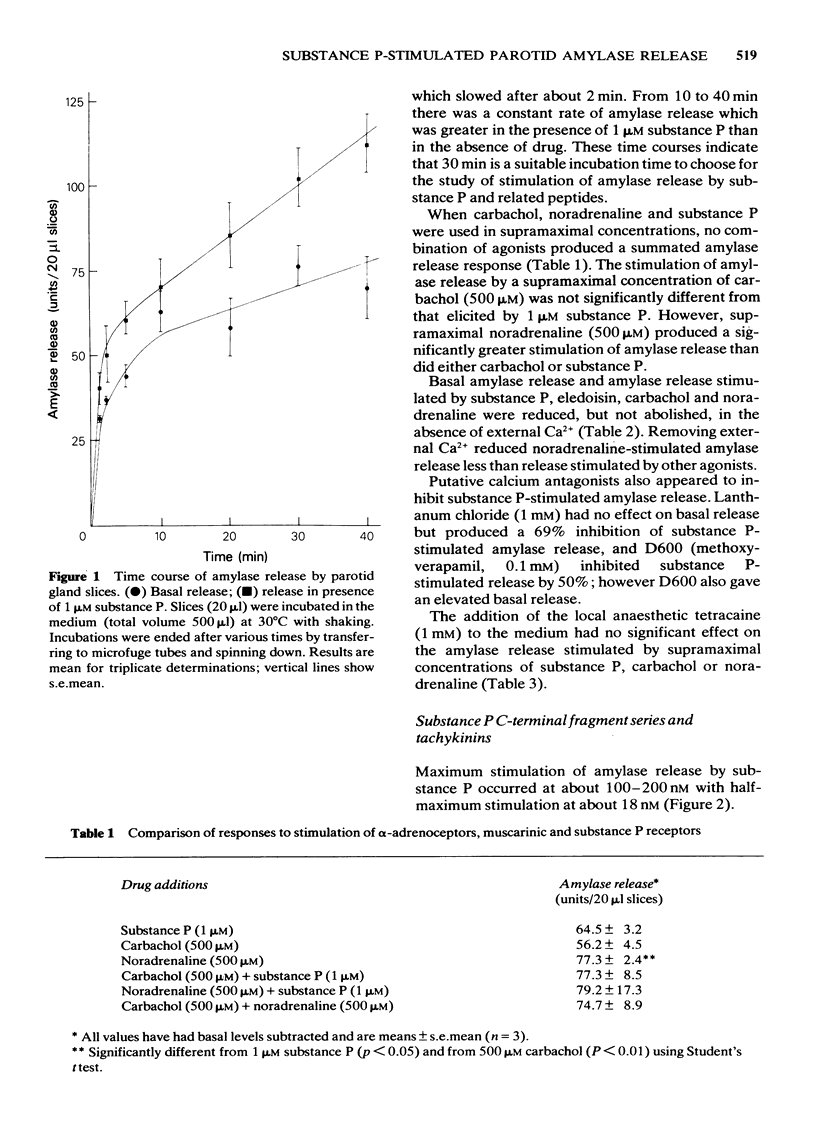
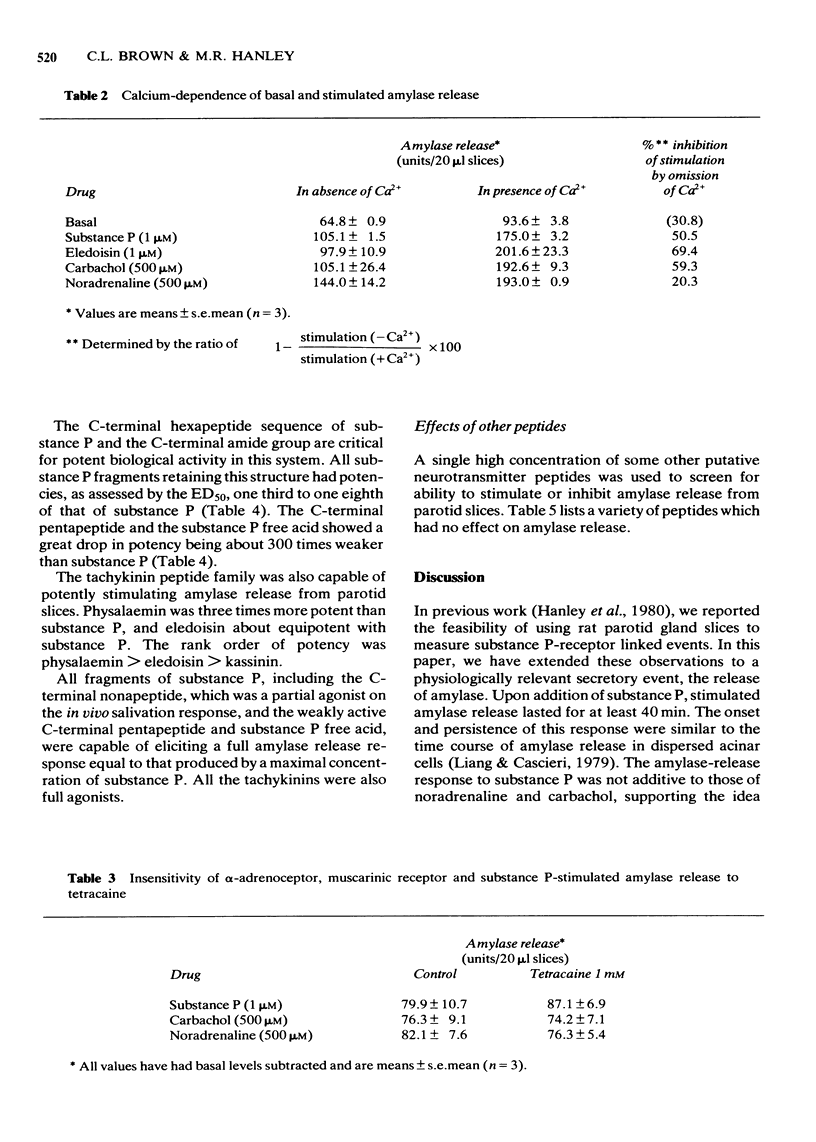
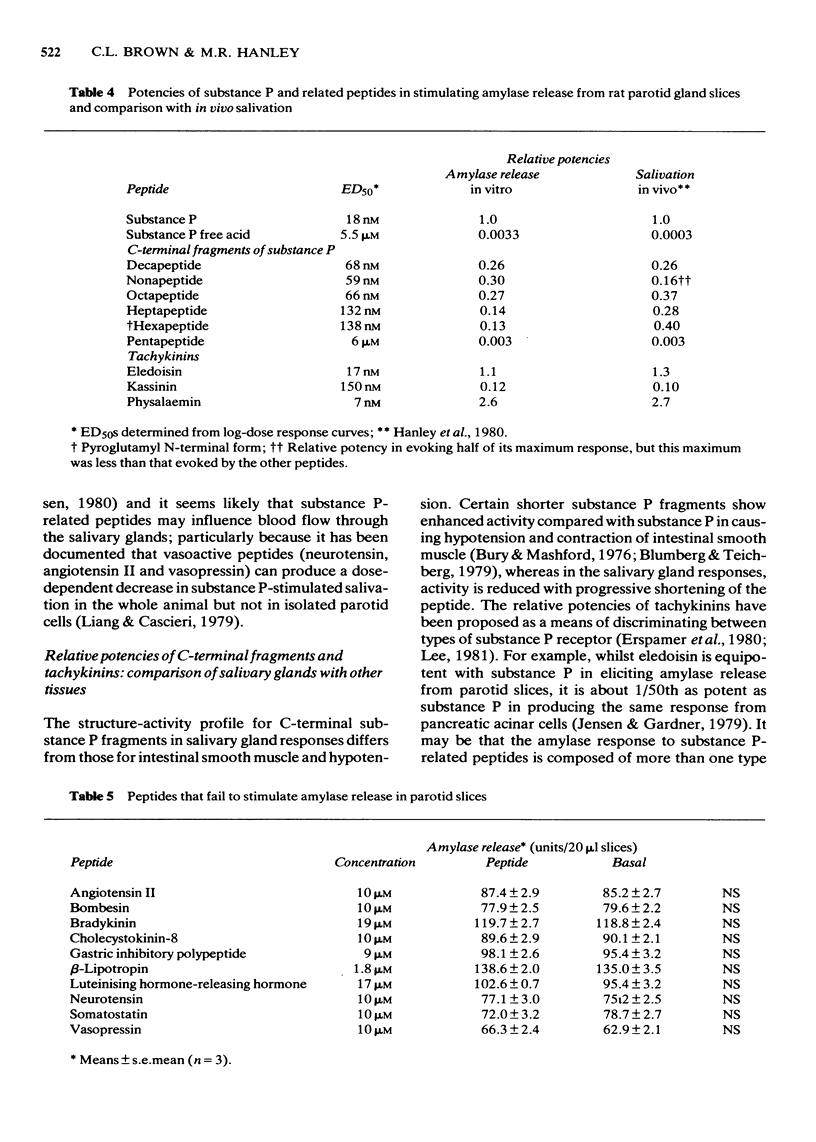
1 The effects of substance P and related peptides on amylase release from rat parotid gland slices have been investigated. 2 Supramaximal concentrations (1 microM) of substance P caused enhancement of amylase release over the basal level within 1 min; this lasted for at least 40 min at 30 degrees C. 3 Substance P-stimulated amylase release was partially dependent on extracellular calcium and could be inhibited by 50% upon removal of extracellular calcium. 4 Substance P stimulated amylase release in a dose-dependent manner with an ED50 of 18 nM. 5 All C-terminal fragments of substance P were less potent than substance P in stimulating amylase release. The C-terminal hexapeptide of substance P was the minimum structure for potent activity in this system, having 1/3 to 1/8 the potency of substance P. There was a dramatic drop in potency for the C-terminal pentapeptide of substance P or substance P free acid. Physalaemin was more potent than substance P (ED50 = 7 nM), eledoisin was about equipotent with substance P (ED50 = 17 nM), and kassinin less potent that substance P (ED50 = 150 nM). 6 The structure-activity profile observed is very similar to that for stimulation of salivation in vivo, indicating that the same receptors are involved in mediating these responses. 7 All the fragments of substance P tested were capable of eliciting a full amylase release response. This indicates that the apparent partial agonist action of the C-terminal nonapeptide fragment on in vivo salivation is not explicable at the receptor level.
Full text
PDF

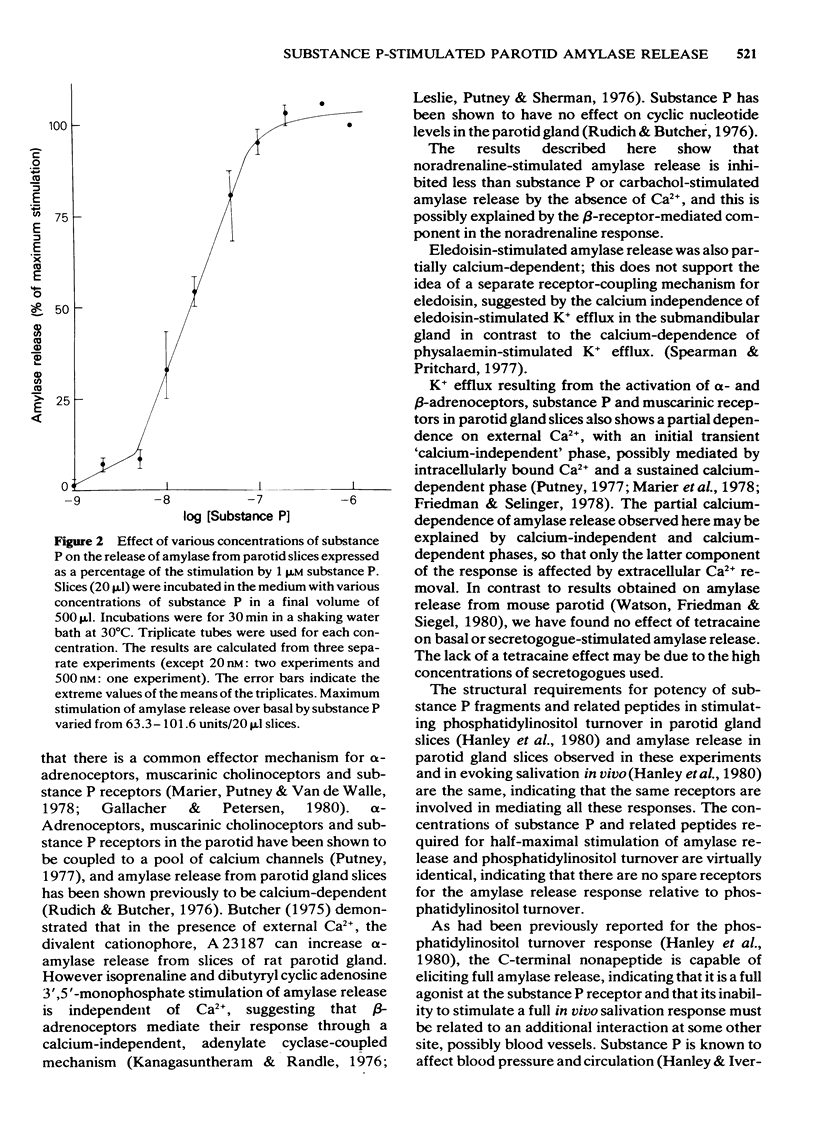

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batzri S., Selinger Z. Enzyme secretion mediated by the epinephrine -receptor in rat parotid slices. Factors governing efficiency of the process. J Biol Chem. 1973 Jan 10;248(1):356–360. [PubMed] [Google Scholar]
- Blumberg S., Teichberg V. I. Biological activity and enzymic degradation of substance P analogs: implications for studies of the substance P receptor. Biochem Biophys Res Commun. 1979 Sep 12;90(1):347–354. doi: 10.1016/0006-291x(79)91631-0. [DOI] [PubMed] [Google Scholar]
- Bury R. W., Mashford M. L. Biological activity of C-terminal partial sequences of substance P. J Med Chem. 1976 Jun;19(6):854–856. doi: 10.1021/jm00228a028. [DOI] [PubMed] [Google Scholar]
- Butcher F. R. The role of calcium and cyclic nucleotides in alpha-amylase release from slices of rat parotid: studies with the divalent cation ionophore A-23187. Metabolism. 1975 Mar;24(3):409–418. doi: 10.1016/0026-0495(75)90120-1. [DOI] [PubMed] [Google Scholar]
- Chang M. M., Leeman S. E. Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J Biol Chem. 1970 Sep 25;245(18):4784–4790. [PubMed] [Google Scholar]
- Chang M. M., Leeman S. E., Niall H. D. Amino-acid sequence of substance P. Nat New Biol. 1971 Jul 21;232(29):86–87. doi: 10.1038/newbio232086a0. [DOI] [PubMed] [Google Scholar]
- Erspamer G. F., Erspamer V., Piccinelli D. Parallel bioassay of physalaemin and kassinin, a tachykinin dodecapeptide from the skin of the African frog Kassina senegalensis. Naunyn Schmiedebergs Arch Pharmacol. 1980 Feb;311(1):61–65. doi: 10.1007/BF00500303. [DOI] [PubMed] [Google Scholar]
- Friedman Z. Y., Selinger Z. A transient release of potassium mediated by the action of substance P on rat parotid slices. J Physiol. 1978 May;278:461–469. doi: 10.1113/jphysiol.1978.sp012317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher D. V., Petersen O. H. Substance P increases membrane conductance in parotid acinar cells. Nature. 1980 Jan 24;283(5745):393–395. doi: 10.1038/283393a0. [DOI] [PubMed] [Google Scholar]
- Hanley M. R., Lee C. M., Michell R. H., Jones L. M. Similar effects of substance P and related peptides on salivation and on phosphatidylinositol turnover in rat salivary glands. Mol Pharmacol. 1980 Jul;18(1):78–83. [PubMed] [Google Scholar]
- Jensen R. T., Gardner J. D. Interaction of physalaemin, substance P, and eledoisin with specific membrane receptors on pancreatic acinar cells. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5679–5683. doi: 10.1073/pnas.76.11.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. M., Michell R. H. Enhanced phosphatidylinositol breakdown as a calcium-indepepdnet response of rat parotid fragments to substance P. Biochem Soc Trans. 1978;6(5):1035–1037. doi: 10.1042/bst0061035. [DOI] [PubMed] [Google Scholar]
- Kanagasuntheram P., Randle P. J. Calcium metabolism and amylase release in rat parotid acinar cells. Biochem J. 1976 Dec 15;160(3):547–564. doi: 10.1042/bj1600547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman S. E., Hammerschlag R. Stimulation of salivary secretion by a factor extracted from hypothalamic tissue. Endocrinology. 1967 Oct;81(4):803–810. doi: 10.1210/endo-81-4-803. [DOI] [PubMed] [Google Scholar]
- Leslie B. A., Putney J. W., Jr, Sherman J. M. alpha-Adrenergic, beta-adrenergic and cholinergic mechanisms for amylase secretion by rat parotid gland in vitro. J Physiol. 1976 Sep;260(2):351–370. doi: 10.1113/jphysiol.1976.sp011519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T., Cascieri M. A. Substance P stimulation of amylase release by isolated parotid cells and inhibition of substance P induction of salivation by vasoactive peptides. Mol Cell Endocrinol. 1979 Sep;15(3):151–162. doi: 10.1016/0303-7207(79)90035-2. [DOI] [PubMed] [Google Scholar]
- Marier S. H., Putney J. W., Jr, Van de Walle C. M. Control of calcium channels by membrane receptors in the rat parotid gland. J Physiol. 1978 Jun;279:141–151. doi: 10.1113/jphysiol.1978.sp012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr Muscarinic, alpha-adrenergic and peptide receptors regulate the same calcium influx sites in the parotid gland. J Physiol. 1977 Jun;268(1):139–149. doi: 10.1113/jphysiol.1977.sp011851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudich L., Butcher F. R. Effect of substance P and eledoisin on K+ efflux, amylase release and cyclic nucleotide levels in slices of rat parotid gland. Biochim Biophys Acta. 1976 Oct 22;444(3):704–711. doi: 10.1016/0304-4165(76)90317-2. [DOI] [PubMed] [Google Scholar]
- Sjödin L., Brodin E., Nilsson G., Conlon T. P. Interaction of substance P with dispersed pancreatic acinar cells from the guinea pig. Binding of radioiodinated peptide. Acta Physiol Scand. 1980 May;109(1):97–105. doi: 10.1111/j.1748-1716.1980.tb06570.x. [DOI] [PubMed] [Google Scholar]
- Spearman T. N., Pritchard E. T. Potassium release from submandibular salivary gland in vitro. Biochim Biophys Acta. 1977 Apr 1;466(1):198–207. doi: 10.1016/0005-2736(77)90219-x. [DOI] [PubMed] [Google Scholar]
- V Euler U. S., Gaddum J. H. An unidentified depressor substance in certain tissue extracts. J Physiol. 1931 Jun 6;72(1):74–87. doi: 10.1113/jphysiol.1931.sp002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson E. L., Friedman J., Siegel I. A. Mediation of beta-adrenergic stimulated amylase release from mouse parotid gland. Life Sci. 1980 Jun 2;26(22):1919–1926. doi: 10.1016/0024-3205(80)90622-0. [DOI] [PubMed] [Google Scholar]


