Abstract
1 The spontaneous and potassium-evoked release of tritium from the rat substantia nigra prelabelled with [3H]-γ-aminobutyric acid [3H]-GABA were assessed in vitro under conditions of superfusion.
2 Kainic acid lesions performed in the right caudate nucleus resulted in a 70% reduction in the ability of the homolateral nigral cells to take up and retain [3H]-GABA when compared with the unlesioned side. The potassium-evoked release of [3H]-GABA remained proportional to the radioactivity retained in the tissue suggesting that the nigral GABA neurones that survived kainic acid treatment were still functional.
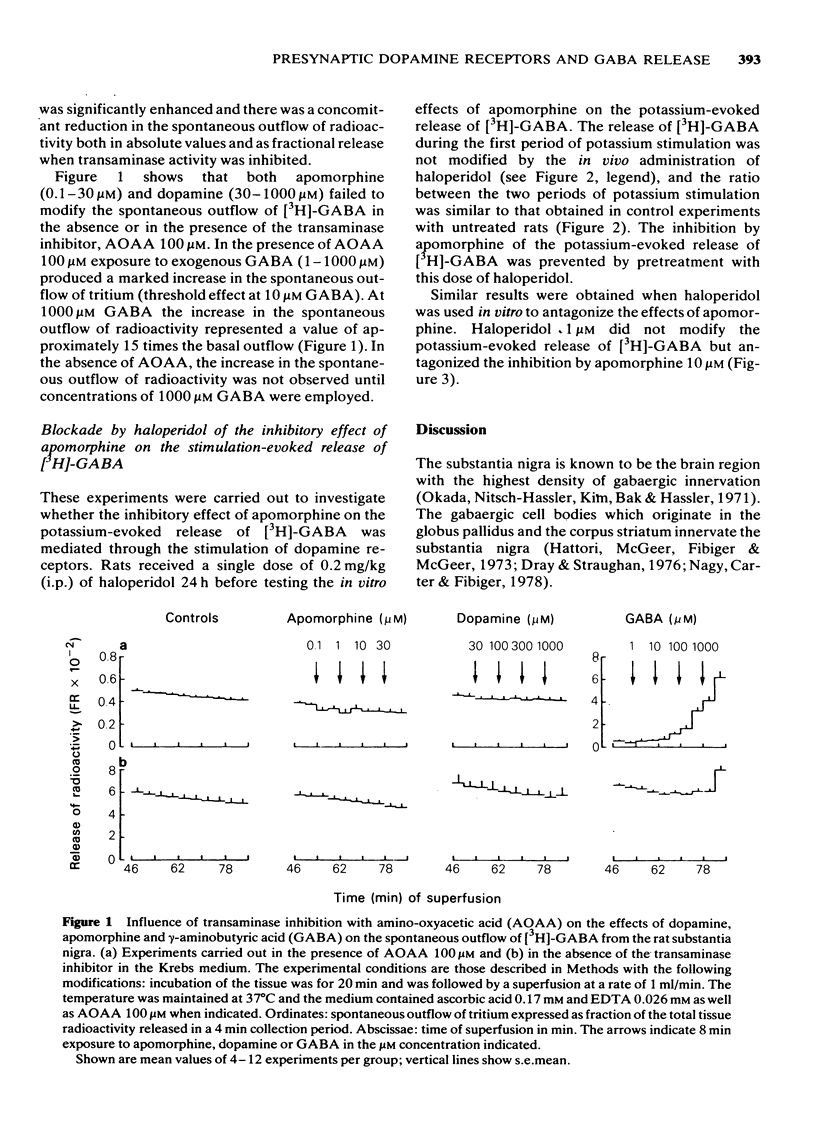
3 The spontaneous outflow of [3H]-GABA was significantly increased by exposure to different concentrations of exogenous GABA (10 to 1000 μM) when amino-oxyacetic acid was present in the incubation medium.
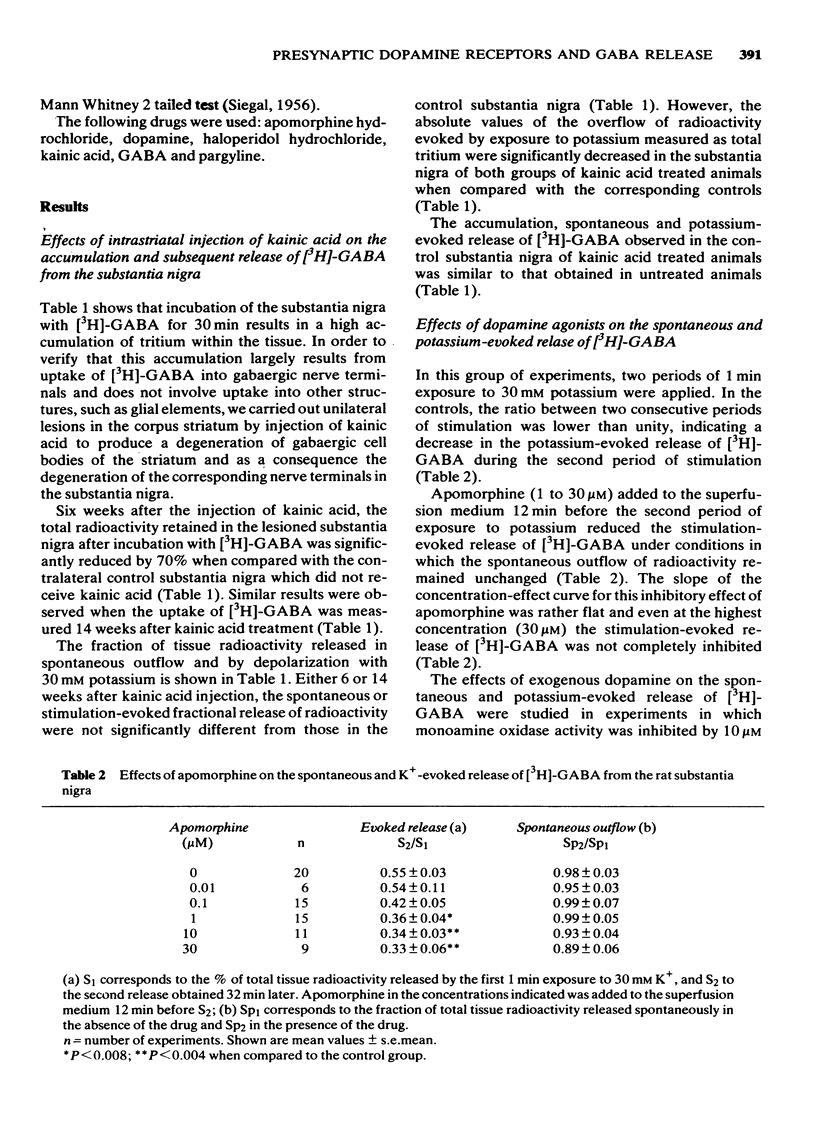
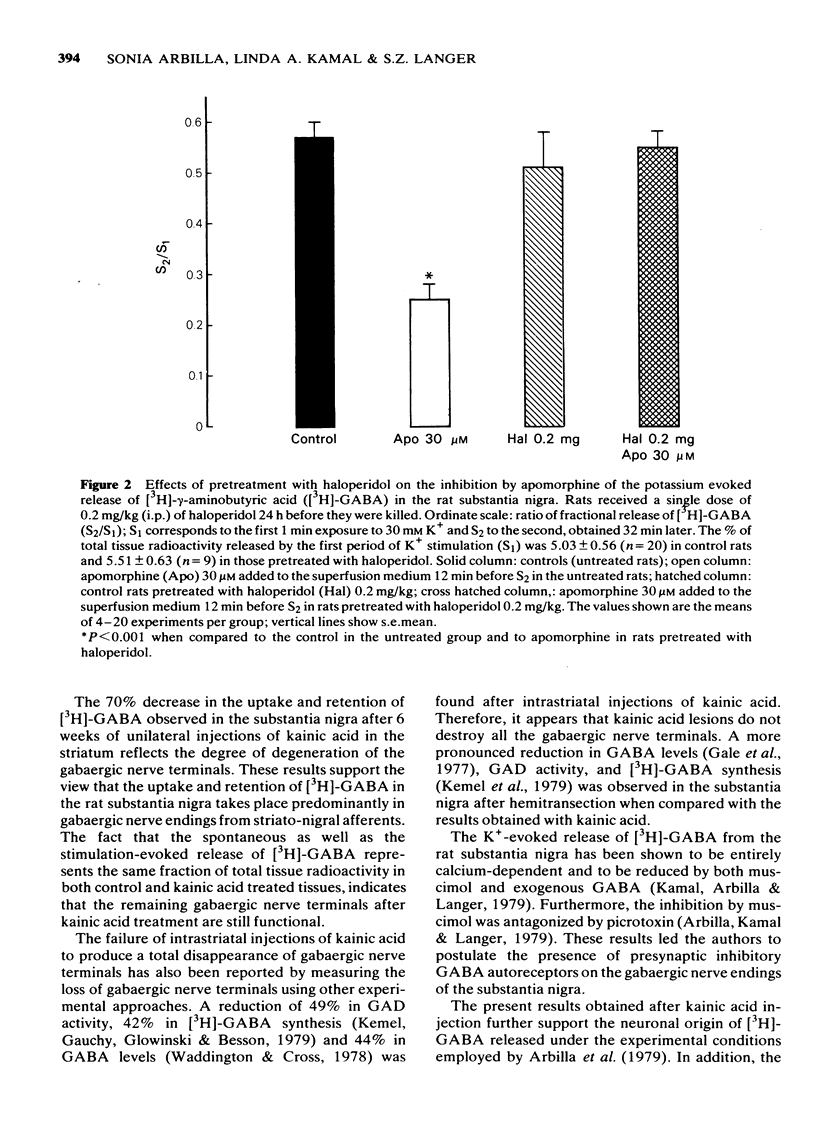
4 Apomorphine in concentrations ranging from 1 to 30 μM inhibited the calcium-dependent release of [3H]-GABA induced by 1 min exposure to 30 mM K+. These concentrations of apomorphine did not affect the spontaneous outflow of radioactivity. In vivo administration of haloperidol 0.2 mg/kg antagonized the in vitro inhibition by apomorphine of the K+-evoked release of [3H]-GABA.
5 The results obtained with apomorphine and haloperidol suggest the presence of presynaptic dopamine-like inhibitory receptors in gabaergic nerve terminals.
6 Dopamine in concentrations ranging up to 300 μM did not modify either the spontaneous or the K+-evoked release of [3H]-GABA from the substantia nigra. These concentrations of dopamine effectively displaced [3H]-dopamine recently taken up into the substantia nigra.
7 Our results do not support the view that dendritic release of dopamine from the substantia nigra might be involved in the physiological modulation of the spontaneous or the stimulation-evoked release of GABA.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Bunney B. S. Dopamine"autoreceptors": pharmacological characterization by microiontophoretic single cell recording studies. Naunyn Schmiedebergs Arch Pharmacol. 1977 Mar;297(1):1–7. doi: 10.1007/BF00508803. [DOI] [PubMed] [Google Scholar]
- Arbilla S., Briley M. S., Dubocovich M. L., Langer S. Z. Neuroleptic binding and their effects on the spontaneous and potassium-evoked release of 3H-dopamine from the striatum and of 3H-noradrenaline from the cerebral cortex. Life Sci. 1978 Oct 30;23(17-18):1775–1780. doi: 10.1016/0024-3205(78)90107-8. [DOI] [PubMed] [Google Scholar]
- Arbilla S., Kamal L., Langer S. Z. Presynaptic GABA autoreceptors on GABAergic nerve endings of the rat substantia nigra. Eur J Pharmacol. 1979 Aug 1;57(2-3):211–217. doi: 10.1016/0014-2999(79)90367-4. [DOI] [PubMed] [Google Scholar]
- Arbilla S., Langer S. Z. Influence of monoamine oxidase inhibition on the release of 3H-dopamine elicited by potassium and by amphetamine from the rat substantia nigra and corpus striatum. Naunyn Schmiedebergs Arch Pharmacol. 1980 Feb;311(1):45–52. doi: 10.1007/BF00500301. [DOI] [PubMed] [Google Scholar]
- Beart P. M., Kuppers D., Louis W. J. Evidence for dopamine receptors on GABA-releasing nerve terminals in rat nucleus accumbens [proceedings]. Br J Pharmacol. 1980 Jan;68(1):160P–161P. [PMC free article] [PubMed] [Google Scholar]
- Beart P. M., McDonald D. Neurochemical studies of the mesolimbic dopaminergic pathway: somatodendritic mechanisms and GABAergic neurones in the rat ventral tegmentum. J Neurochem. 1980 Jun;34(6):1622–1629. doi: 10.1111/j.1471-4159.1980.tb11253.x. [DOI] [PubMed] [Google Scholar]
- Dismukes K., Mulder A. H. Effects of neuroleptics on release of 3H-dopamine from slices of rat corpus striatum. Naunyn Schmiedebergs Arch Pharmacol. 1977 Mar;297(1):23–29. doi: 10.1007/BF00508806. [DOI] [PubMed] [Google Scholar]
- Dray A., Straughan D. W. Synaptic mechanisms in the substantia nigra. J Pharm Pharmacol. 1976 Apr;28(4 Suppl):400–405. doi: 10.1111/j.2042-7158.1976.tb04187.x. [DOI] [PubMed] [Google Scholar]
- Farnebo L. O., Hamberger B. Drug-induced changes in the release of 3 H-monoamines from field stimulated rat brain slices. Acta Physiol Scand Suppl. 1971;371:35–44. doi: 10.1111/j.1748-1716.1971.tb05213.x. [DOI] [PubMed] [Google Scholar]
- Fuder H., Muscholl E. The effect of dopamine on the overflow of endogenous noradrenaline from the perfused rabbit heart evoked by sympathetic nerve stimulation. Naunyn Schmiedebergs Arch Pharmacol. 1978 Nov;305(2):109–115. doi: 10.1007/BF00508279. [DOI] [PubMed] [Google Scholar]
- Gale K., Guidotti A., Costa E. Dopamine-sensitive adenylate cyclase: location in substantia nigra. Science. 1977 Feb 4;195(4277):503–505. doi: 10.1126/science.13499. [DOI] [PubMed] [Google Scholar]
- Hattori T., McGeer P. L., Fibiger H. C., McGeer E. G. On the source of GABA-containing terminals in the substantia nigra. Electron microscopic autoradiographic and biochemical studies. Brain Res. 1973 May 17;54:103–114. doi: 10.1016/0006-8993(73)90037-1. [DOI] [PubMed] [Google Scholar]
- Kamal L. A., Arbilla S., Langer S. Z. Presynaptic modulation of the release of dopamine from the rabbit caudate nucleus: differences between electrical stimulation, amphetamine and tyramine. J Pharmacol Exp Ther. 1981 Mar;216(3):592–598. [PubMed] [Google Scholar]
- Kebabian J. W., Saavedra J. M. Dopamine-sensitive adenylate cyclase occurs in a region of substantia nigra containing dopaminergic dendrites. Science. 1976 Aug 20;193(4254):683–685. doi: 10.1126/science.181842. [DOI] [PubMed] [Google Scholar]
- Kemel M. L., Gauchy C., Glowinski J., Besson M. J. Spontaneous and potassium-evoked release of 3H-GABA newly synthesized from 3H-glutamine in slices of the rat substantia nigra. Life Sci. 1979 Jun 4;24(23):2139–2149. doi: 10.1016/0024-3205(79)90112-7. [DOI] [PubMed] [Google Scholar]
- Langer S. Z. Sixth gaddum memorial lecture, National Institute for Medical Research, Mill Hill, January 1977. Presynaptic receptors and their role in the regulation of transmitter release. Br J Pharmacol. 1977 Aug;60(4):481–497. doi: 10.1111/j.1476-5381.1977.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre-Borg F., Cavero I. Stimulation of peripheral dopamine receptors in rats: a mechanism for novel antihypertensive agents. Clin Sci (Lond) 1980 Dec;59 (Suppl 6):291s–294s. doi: 10.1042/cs059291s. [DOI] [PubMed] [Google Scholar]
- Lokhandwala M. F., Jandhyala B. S. The role of sympathetic nervous system in the vascular actions of dopamine. J Pharmacol Exp Ther. 1979 Jul;210(1):120–126. [PubMed] [Google Scholar]
- Long J. P., Heintz S., Cannon J. G., Kim J. Inhibition of the sympathetic nervous system by 5,6-dihydroxy-2-dimethylamino tetralin (M-7), apomorphine and dopamine. J Pharmacol Exp Ther. 1975 Feb;192(2):336–342. [PubMed] [Google Scholar]
- Okada Y., Nitsch-Hassler C., Kim J. S., Bak I. J., Hassler R. Role of -aminobutyric acid (GABA) in the extrapyramidal motor system. 1. Regional distribution of GABA in rabbit, rat, guinea pig and baboon CNS. Exp Brain Res. 1971 Nov 30;13(5):514–518. doi: 10.1007/BF00234282. [DOI] [PubMed] [Google Scholar]
- Pelayo F., Dubocovich M. L., Langer S. Z. Inhibition of neuronal uptake reduces the presynaptic effects of clonidine but not of alpha-methylnoradrenaline on the stimulation-evoked release of 3H-noradrenaline from rat occipital cortex slices. Eur J Pharmacol. 1980 Jun 13;64(2-3):143–155. doi: 10.1016/0014-2999(80)90037-0. [DOI] [PubMed] [Google Scholar]
- Phillipson O. T., Horn A. S. Substantia nigra of the rat contains a dopamine sensitive adenylate cyclase. Nature. 1976 Jun 3;261(5559):418–420. doi: 10.1038/261418a0. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Iversen L. L., Jessell T. M. Dopamine selectively increases 3H-GABA release from slices of rat substantia nigra in vitro. Nature. 1977 Aug 18;268(5621):652–654. doi: 10.1038/268652a0. [DOI] [PubMed] [Google Scholar]
- Seeman P., Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975 Jun 20;188(4194):1217–1219. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- Starke K., Reimann W., Zumstein A., Hertting G. Effect of dopamine receptor agonists and antagonists on release of dopamine in the rabbit caudate nucleus in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1978 Oct;305(1):27–36. doi: 10.1007/BF00497003. [DOI] [PubMed] [Google Scholar]
- Waddington J. L., Cross A. J. Denervation supersensitivity in the striatonigral GABA pathway. Nature. 1978 Dec 7;276(5688):618–620. doi: 10.1038/276618a0. [DOI] [PubMed] [Google Scholar]
- van der Heyden J. A., Venema K., Korf J. Biphasic and opposite effects of dopamine and apomorphine on endogenous GABA release in the rat substantia nigra. J Neurochem. 1980 Jan;34(1):119–125. doi: 10.1111/j.1471-4159.1980.tb04629.x. [DOI] [PubMed] [Google Scholar]


