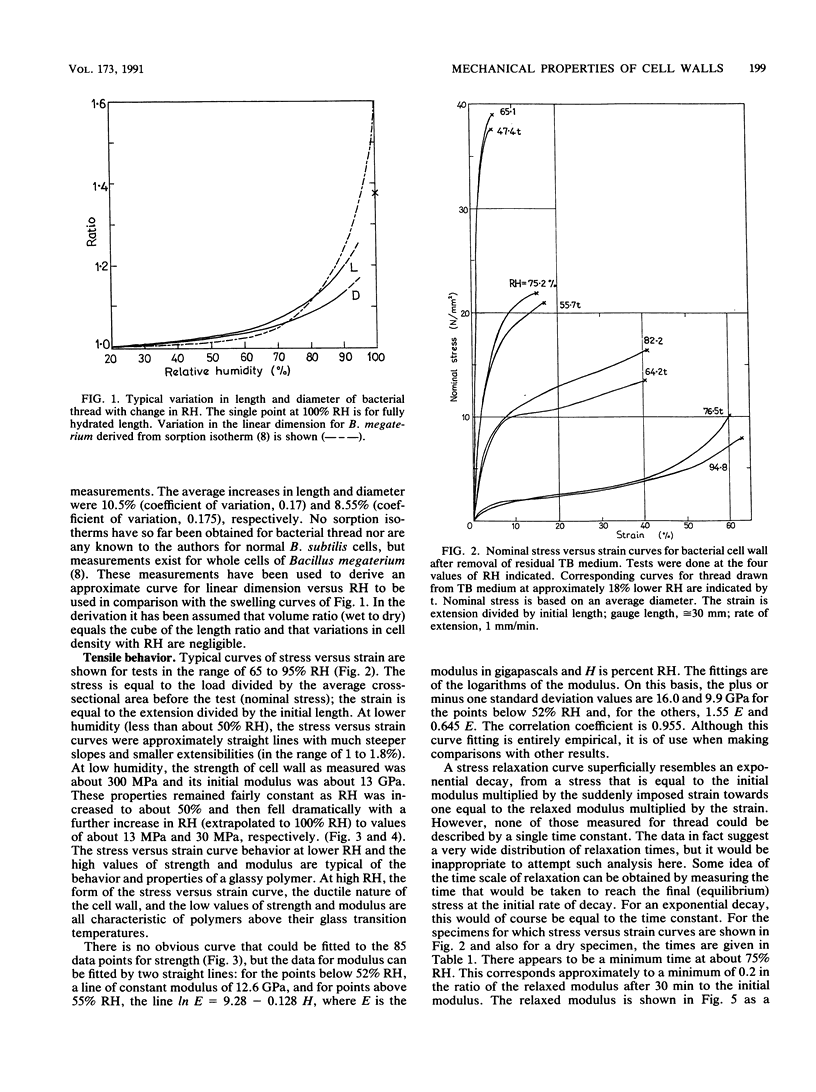
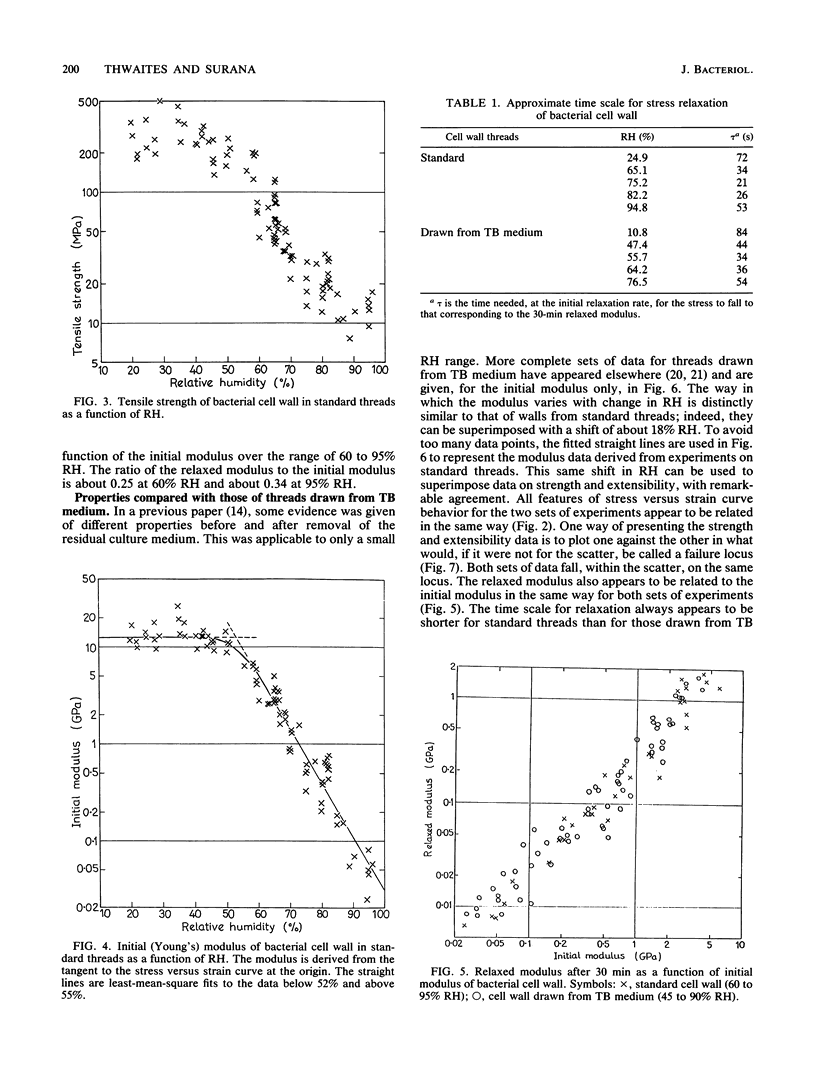
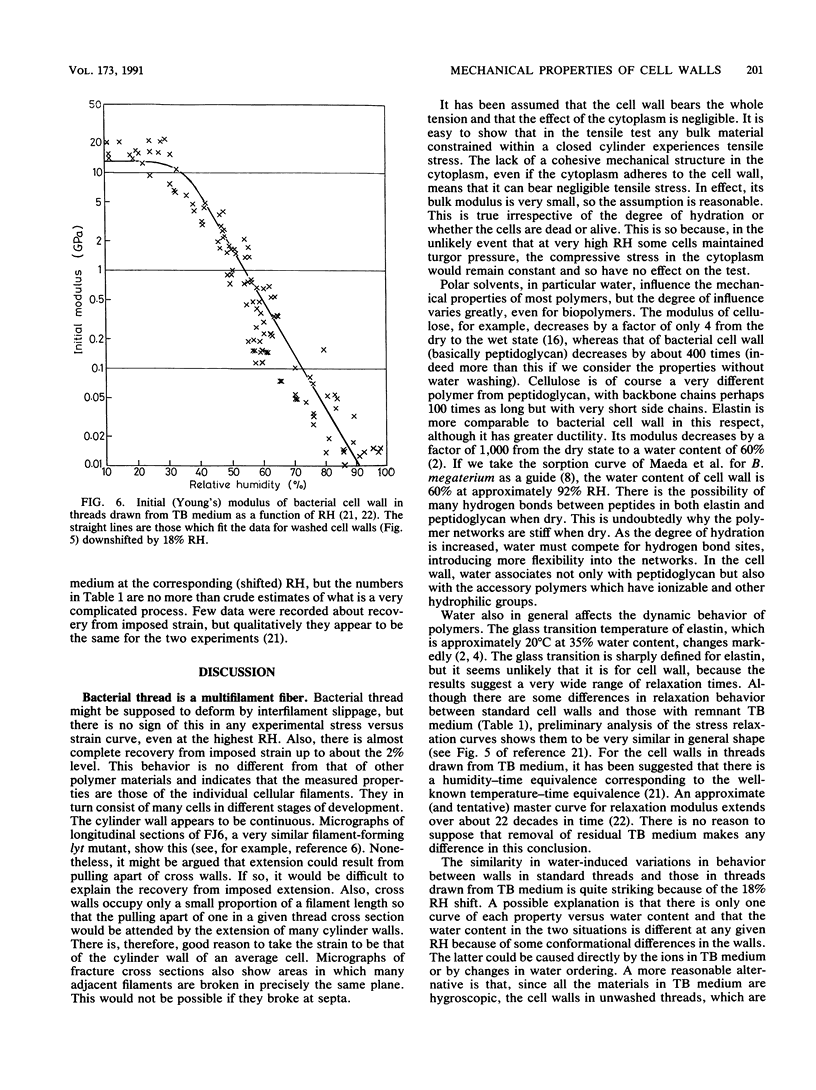
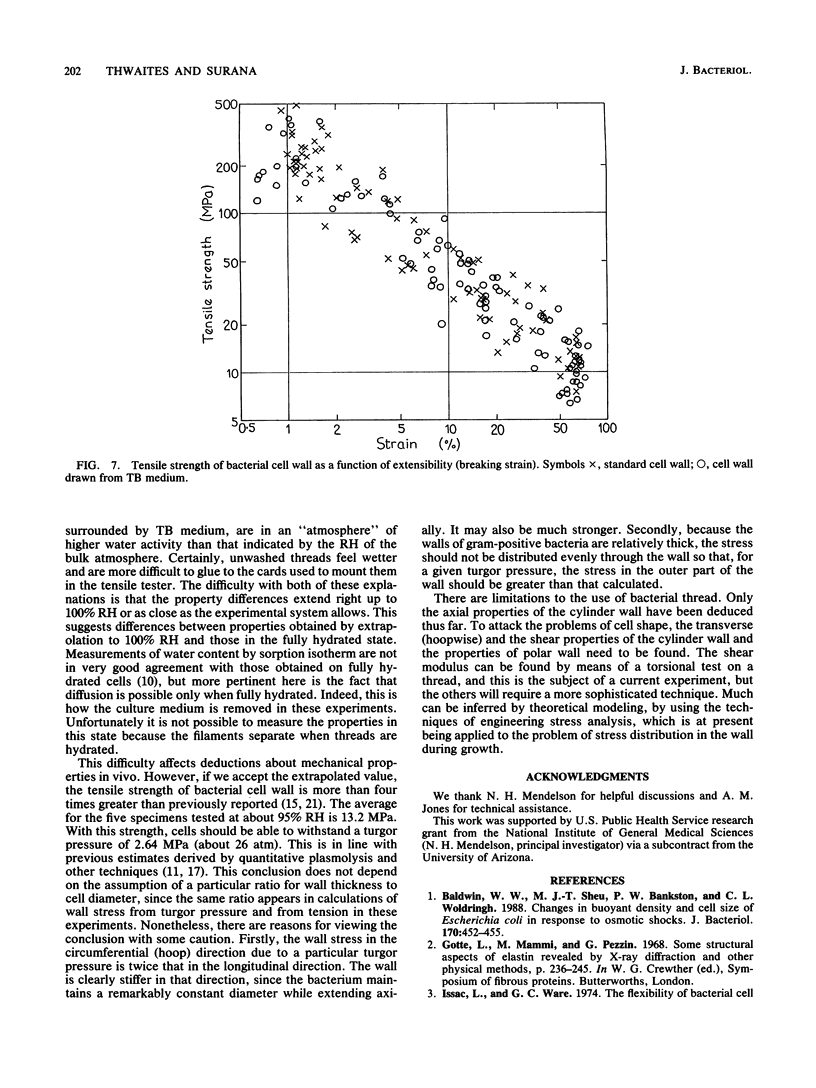
Abstract
Experiments are described in which the tensile strength, the initial (Youngs') modulus, and other mechanical properties of the bacterial cell wall were obtained as functions of relative humidity (RH) in the range of 20 to 95%. These properties were deduced from tensile tests on bacterial thread, a fiber consisting of many highly aligned cells of Bacillus subtilis, from which residual culture medium had been removed by immersion in water. Reasons are given to support the idea that the mechanical properties of bacterial thread relate directly to those of the cylinder wall and that they are not influenced by septa, cytoplasm, or the thread assembly. The data show that the cell wall, like many other heteropolymers, is visco-elastic. When dry, it behaves like a glassy polymer with a tensile strength of about 300 MPa and a modulus of about 13 GPa. When wet, its behavior is more like a rubbery polymer with a tensile strength of about 13 MPa and a modulus of about 30 MPa. Thus, the cell wall is stronger than previously reported. Walls of this strength would be able to bear a turgor pressure of 2.6 MPa (about 26 atm). The dynamic behavior suggests a wide range of relaxation times. The way in which mechanical behavior depends strongly on humidity is discussed in terms of possible hydrogen bond density and the ordering of water molecules. Cell walls in threads containing residual culture medium TB are, except at low RH, 10 times more flexible and about 4 times less strong. All of their mechanical properties appear to vary with change in RH in a manner similar to those of walls from which the culture medium has been washed, but with a downshift of about 18% RH.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin W. W., Sheu M. J., Bankston P. W., Woldringh C. L. Changes in buoyant density and cell size of Escherichia coli in response to osmotic shocks. J Bacteriol. 1988 Jan;170(1):452–455. doi: 10.1128/jb.170.1.452-455.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac L., Ware G. C. The flexibility of bacterial cell walls. J Appl Bacteriol. 1974 Sep;37(3):335–339. doi: 10.1111/j.1365-2672.1974.tb00448.x. [DOI] [PubMed] [Google Scholar]
- KNAYSI G., HILLIER J., FABRICANT C. The cytology of an avian strain of Mycobacterium tuberculosis studied with the electron and light microscopes. J Bacteriol. 1950 Oct;60(4):423–447. doi: 10.1128/jb.60.4.423-447.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakivaya S. R., Hoeve C. A. The glass point of elastin. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3505–3507. doi: 10.1073/pnas.72.9.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L., Burdett I. D. Normal pole formation during total inhibition of wall synthesis of Bacillus subtilis. J Gen Microbiol. 1986 Dec;132(12):3441–3449. doi: 10.1099/00221287-132-12-3441. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Lane S. L., Miller J. A., Nickens D. G. Contraction of filaments of Escherichia coli after disruption of cell membrane by detergent. J Bacteriol. 1987 May;169(5):1979–1984. doi: 10.1128/jb.169.5.1979-1984.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E., Carstensen E. L. Electric conductivity and internal osmolality of intact bacterial cells. J Bacteriol. 1973 Mar;113(3):1198–1206. doi: 10.1128/jb.113.3.1198-1206.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E. Salt-induced contraction of bacterial cell walls. J Bacteriol. 1968 Mar;95(3):775–781. doi: 10.1128/jb.95.3.775-781.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H., Favre D., Thwaites J. J. Twisted states of Bacillus subtilis macrofibers reflect structural states of the cell wall. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3562–3566. doi: 10.1073/pnas.81.11.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H., Thwaites J. J. Cell wall mechanical properties as measured with bacterial thread made from Bacillus subtilis. J Bacteriol. 1989 Feb;171(2):1055–1062. doi: 10.1128/jb.171.2.1055-1062.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou L. T., Marquis R. E. Electromechanical interactions in cell walls of gram-positive cocci. J Bacteriol. 1970 Jan;101(1):92–101. doi: 10.1128/jb.101.1.92-101.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites J. J., Mendelson N. H. Biomechanics of bacterial walls: studies of bacterial thread made from Bacillus subtilis. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2163–2167. doi: 10.1073/pnas.82.7.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites J. J., Mendelson N. H. Mechanical properties of peptidoglycan as determined from bacterial thread. Int J Biol Macromol. 1989 Aug;11(4):201–206. doi: 10.1016/0141-8130(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Thwaites J. J., Surana U. C., Jones A. M. Mechanical properties of Bacillus subtilis cell walls: effects of ions and lysozyme. J Bacteriol. 1991 Jan;173(1):204–210. doi: 10.1128/jb.173.1.204-210.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


