Abstract
1 The submucous plexus-longitudinal muscularis mucosae preparation of the guinea-pig oesophagus was used to study the actions of catecholamines on the twitch responses to electrical stimulation.
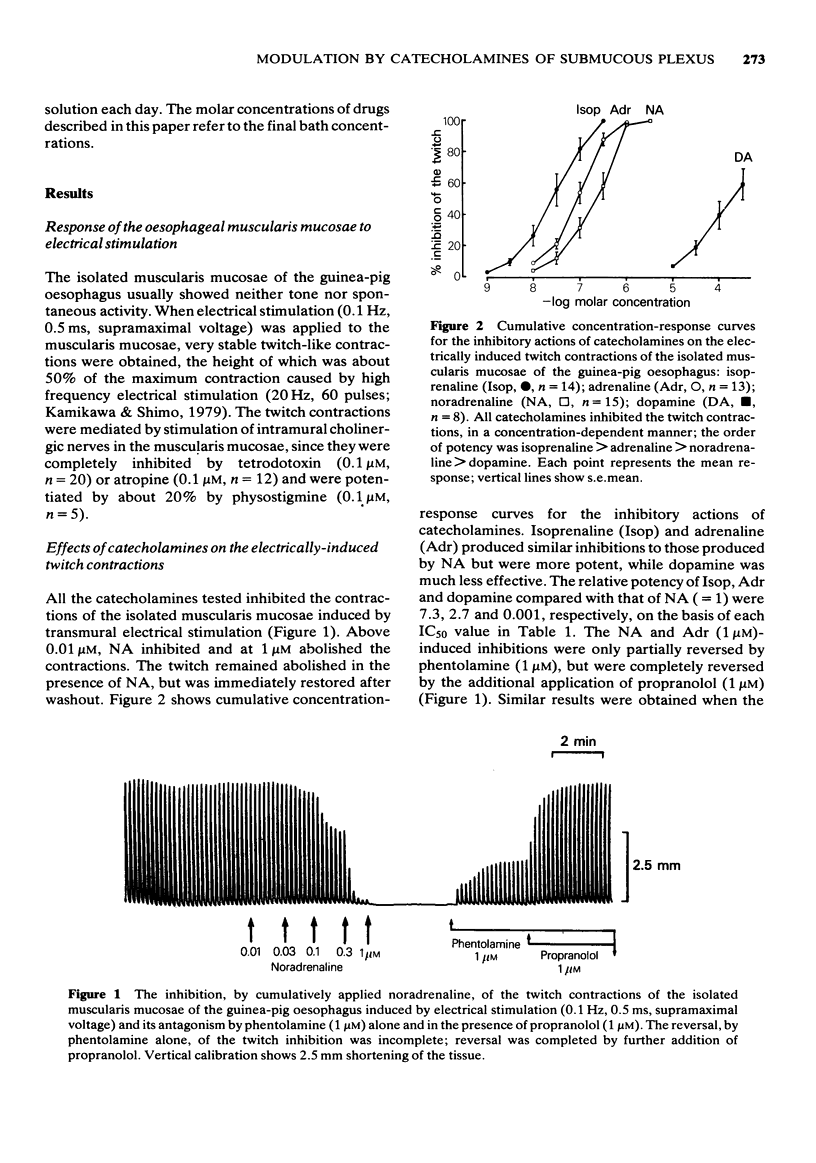
2 When the preparation was stimulated coaxially (0.1 Hz, 0.5 ms, supramaximal voltage), stable twitch-like contractions were obtained. These were abolished by tetrodotoxin (0.1 μM) and atropine (0.1 μM), potentiated by physostigmine (0.1 μM), and were mediated presumably by stimulation of intramural cholinergic nerves.
3 The twitch contractions of the muscularis mucosae were inhibited by catecholamines, in a concentration-dependent manner. The order of potency was isoprenaline > adrenaline > noradrenaline > dopamine.
4 The inhibitory actions of noradrenaline (1 μM) and adrenaline (1 μM) were partly reversed by phentolamine (1 μM) or by propranolol (1 μM), and completely abolished by both antagonists together. The inhibitory effect of dopamine (300 μM) was largely reversed by phentolamine (1 μM), but not by propranolol (1 μM), while the inhibitory action of isoprenaline was competitively antagonized only by propranolol (pA2 of 7.6).
5 The contraction of the muscularis mucosae to exogenously applied acetylcholine (ACh, 20 nM) which was comparable in magnitude with that to electrical stimulation was also inhibited by isoprenaline (0.1 μM), adrenaline (1 μM) and noradrenaline (1 μM), but not by dopamine (300 μM). In the presence of propranolol (1 μM), noradrenaline, adrenaline and dopamine potentiated the ACh-induced contraction, while the effect of isoprenaline was mainly antagonized. The potentiating effects were antagonized by further treatment with phentolamine (1 μM).
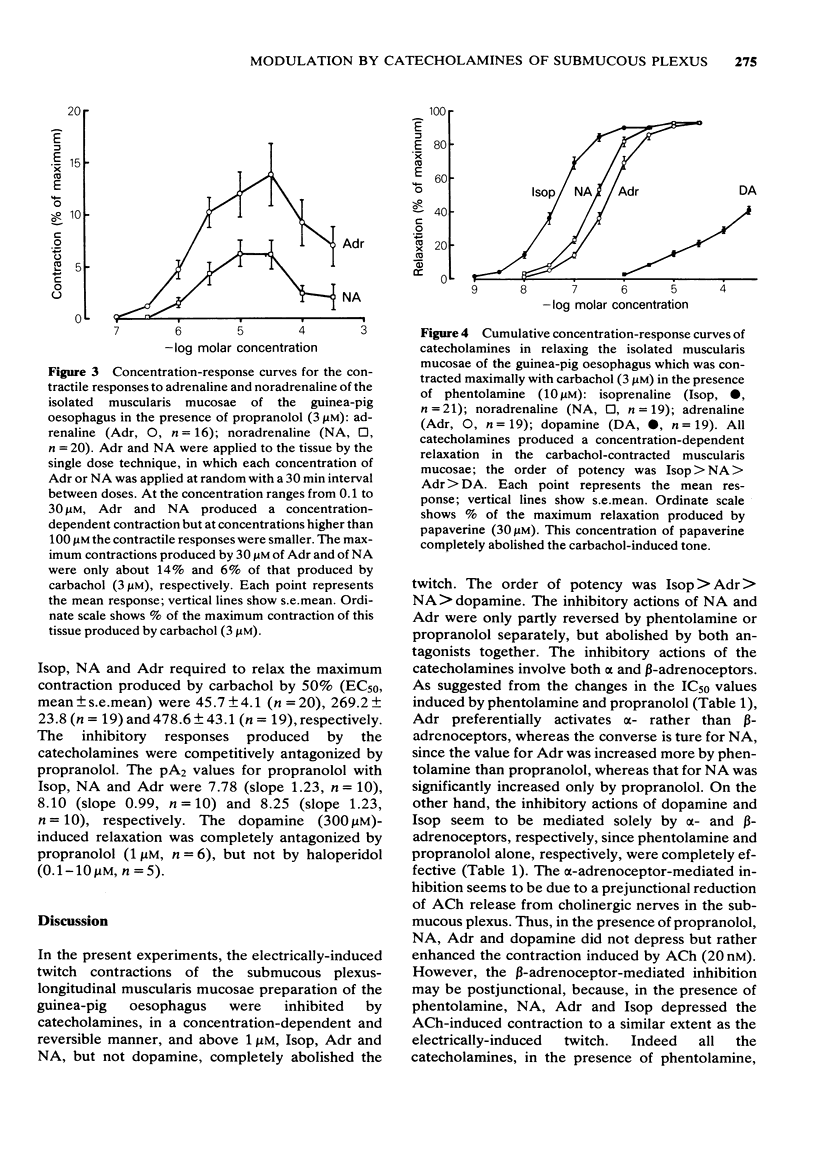
6 Adrenaline, noradrenaline and dopamine but not isoprenaline, produced a weak contraction of the longitudinal muscularis mucosae in the presence of propranolol (3 μM). The contractile responses were completely inhibited by phentolamine (3 μM). Tone in the muscularis mucosae induced by carbachol (3 μM) in the presence of phentolamine (10 μM) was inhibited by catecholamines, in a concentration-dependent manner, an effect that was competitively antagonized by propranolol.
7 In the submucous plexus-longitudinal muscularis mucosae preparation of the guinea-pig oesophagus there are three types of adrenoceptor, inhibitory prejunctional α-adrenoceptors, excitatory postjunctional α-adrenoceptors and inhibitory postjunctional β-adrenoceptors, and cholinergic neurotransmission is inhibited by catecholamines acting at both prejunctional α- and postjunctional β-adrenoceptors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey D. M. The action of sympathomimetic amines on circular and longitudinal smooth muscle from the isolated oesophagus of the guinea-pig. J Pharm Pharmacol. 1965 Dec;17(12):782–787. doi: 10.1111/j.2042-7158.1965.tb07606.x. [DOI] [PubMed] [Google Scholar]
- Bauer V. Distribution and types of adrenoceptors in the guinea-pig ileum: the action of alpha- and beta-adrenoceptor agonists. Br J Pharmacol. 1981 Feb;72(2):201–210. doi: 10.1111/j.1476-5381.1981.tb09114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M., Furness J. B. The simultaneous demonstration of adrenergic fibres and enteric ganglion cells. Histochem J. 1973 Jul;5(4):343–349. doi: 10.1007/BF01004802. [DOI] [PubMed] [Google Scholar]
- Cox B., Ennis C. Mechanism of action of dopamine on the guinea-pig gastro-oesophageal junction in vitro. Br J Pharmacol. 1980;71(1):177–184. doi: 10.1111/j.1476-5381.1980.tb10923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew G. M. Pharmacological characterization of the presynaptic alpha-adrenoceptors regulating cholinergic activity in the guinea-pig ileum. Br J Pharmacol. 1978 Oct;64(2):293–300. doi: 10.1111/j.1476-5381.1978.tb17303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B., Costa M. Types of nerves in the enteric nervous system. Neuroscience. 1980;5(1):1–20. doi: 10.1016/0306-4522(80)90067-6. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., Khoyi M. A. The site and receptors responsible for the inhibition by sympathetic nerves of intestinal smooth muscle and its parasympathetic motor nerves. J Physiol. 1977 Jun;267(3):767–789. doi: 10.1113/jphysiol.1977.sp011837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., McKirdy H. C. Synaptic potentials recorded from neurones of the submucous plexus of guinea-pig small intestine. J Physiol. 1975 Jul;249(2):369–385. doi: 10.1113/jphysiol.1975.sp011020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Silinsky E. M. Some effects of 5-hydroxytryptamine, dopamine and noradrenaline on neurones in the submucous plexus of guinea-pig small intestine. J Physiol. 1975 Oct;251(3):817–832. doi: 10.1113/jphysiol.1975.sp011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollands B. C., Vanov S. Localization of catechol amines in visceral organs and ganglia of the rat, guinea-pig and rabbit. Br J Pharmacol Chemother. 1965 Oct;25(2):307–316. doi: 10.1111/j.1476-5381.1965.tb02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobowitz D. Histochemical studies of the autonomic innervation of the gut. J Pharmacol Exp Ther. 1965 Sep;149(3):358–364. [PubMed] [Google Scholar]
- Kamikawa Y., Shimo Y. Antagonistic effect of compound 48/80 on the inhibitory actions of morphine and methionine-enkephalin on electrically-induced contractions of the guinea-pig ileum. Br J Pharmacol. 1978 Dec;64(4):511–518. doi: 10.1111/j.1476-5381.1978.tb17312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikawa Y., Shimo Y. Cholinergic and adrenergic innervations of the muscularis mucosae in guinea-pig esophagus. Arch Int Pharmacodyn Ther. 1979 Apr;238(2):220–232. [PubMed] [Google Scholar]
- Kosterlitz H. W., Lydon R. J., Watt A. J. The effects of adrenaline, noradrenaline and isoprenaline on inhibitory alpha- and beta-adrenoceptors in the longitudinal muscle of the guinea-pig ileum. Br J Pharmacol. 1970 Jun;39(2):398–413. doi: 10.1111/j.1476-5381.1970.tb12903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNRO A. F. The effect of adrenaline on the guinea-pig intestine. J Physiol. 1951 Jan;112(1-2):84–94. doi: 10.1113/jphysiol.1951.sp004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORBERG K. A. ADRENERGIC INNERVATION OF THE INTESTINAL WALL STUDIED BY FLUORESCENCE MICROSCOPY. Int J Neuropharmacol. 1964 Sep;3:379–382. doi: 10.1016/0028-3908(64)90067-x. [DOI] [PubMed] [Google Scholar]
- Nozdrachev A. D., Vataev S. I. Neuronal electrical activity in the submucosal plexus of the cat small intestine. J Auton Nerv Syst. 1981 Feb;3(1):45–53. doi: 10.1016/0165-1838(81)90029-1. [DOI] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S. The inhibitory action of noradrenaline and adrenaline on acetylcholine output by guinea-pig ileum longitudinal muscle strip. Br J Pharmacol. 1969 Jan;35(1):10–28. doi: 10.1111/j.1476-5381.1969.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansy M. F., Martin J. S., Landin W. E., Kendall F. M. Localization of isoproterenol-induced contractions of canine small intestine. J Pharm Sci. 1979 Jan;68(1):127–128. doi: 10.1002/jps.2600680147. [DOI] [PubMed] [Google Scholar]
- WALDER D. N. The muscularis mucosae of the human stomach. J Physiol. 1953 May 28;120(3):365–372. doi: 10.1113/jphysiol.1953.sp004900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON A. B. BETA SYMPATHETIC INHIBITORY RECEPTORS IN THE SMALL INTESTINE OF THE GUINEA-PIG. J Pharm Pharmacol. 1964 Dec;16:834–835. doi: 10.1111/j.2042-7158.1964.tb07421.x. [DOI] [PubMed] [Google Scholar]
- Wikberg J. Differentiation between pre- and postjunctional alpha-receptors in guinea pig ileum and rabbit aorta. Acta Physiol Scand. 1978 Jul;103(3):225–239. doi: 10.1111/j.1748-1716.1978.tb06210.x. [DOI] [PubMed] [Google Scholar]
- Wikberg J. Localization of adrenergic receptors in guinea pig ileum and rabbit jejunum to cholinergic neurons and to smooth muscle cells. Acta Physiol Scand. 1977 Feb;99(2):190–207. doi: 10.1111/j.1748-1716.1977.tb10370.x. [DOI] [PubMed] [Google Scholar]


