Abstract
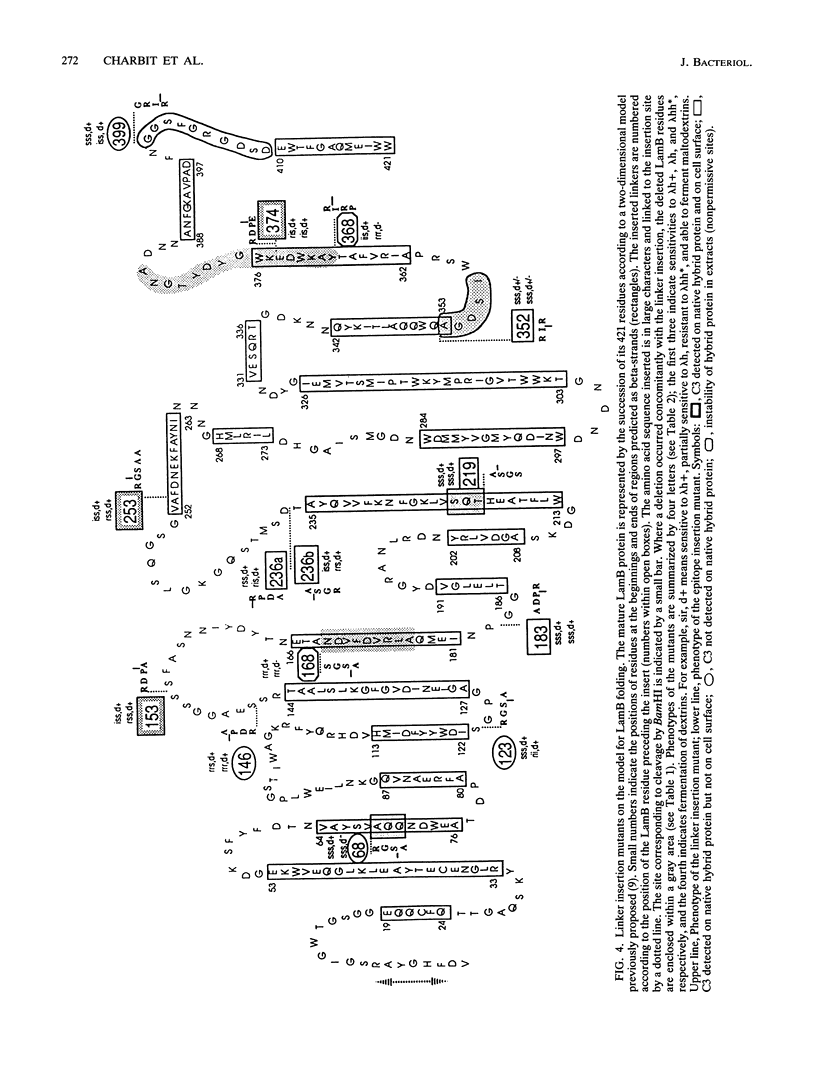
We are developing a genetic approach to study with a single antibody the folding and topology of LamB, an integral outer membrane protein from Escherichia coli K-12. This approach consists of inserting the same reporter foreign antigenic determinant (the C3 epitope from poliovirus) at different sites of LamB so that the resulting hybrid proteins have essentially kept the in vivo biological properties of LamB and therefore its cellular location and structure; the corresponding sites are called permissive sites. A specific monoclonal antibody can then be used to examine the position of the reporter epitope with respect to the protein and the membrane. We present an improved and efficient procedure that led us to identify eight new permissive sites in LamB. These sites appear to be distributed on both sides of the membrane. At one of them (after residue 253), the C3 epitope was detected on intact bacteria, providing the first direct argument for exposure of the corresponding LamB region at the cell surface. At this site as well as at four others (after residues 183, 219, 236, and 352), the C3 epitope could be detected with the C3 monoclonal antibody at the surface of the extracted trimeric LamB-C3 hybrid proteins. We provide a number of convergent arguments showing that the hybrid proteins are not strongly distorted with respect to the wild-type protein so that the conclusions drawn are also valid for this protein. These conclusions are essentially in agreement with the proposed folding model for the LamB protein. They agree, in particular, with the idea that regions 183 and 352 are exposed to the periplasm. In addition, they suggest that region 236 is buried at the external face of the outer membrane and that region 219 is exposed to the periplasm. Including the 3 sites previously determined, 11 permissive sites are now available in LamB, including 3 at the cell surface and most probably at least 3 in the periplasm. We discuss the nature of such sites, the generalization of this approach to other proteins, and possible applications.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agterberg M., Adriaanse H., Tommassen J. Use of outer membrane protein PhoE as a carrier for the transport of a foreign antigenic determinant to the cell surface of Escherichia coli K-12. Gene. 1987;59(1):145–150. doi: 10.1016/0378-1119(87)90275-7. [DOI] [PubMed] [Google Scholar]
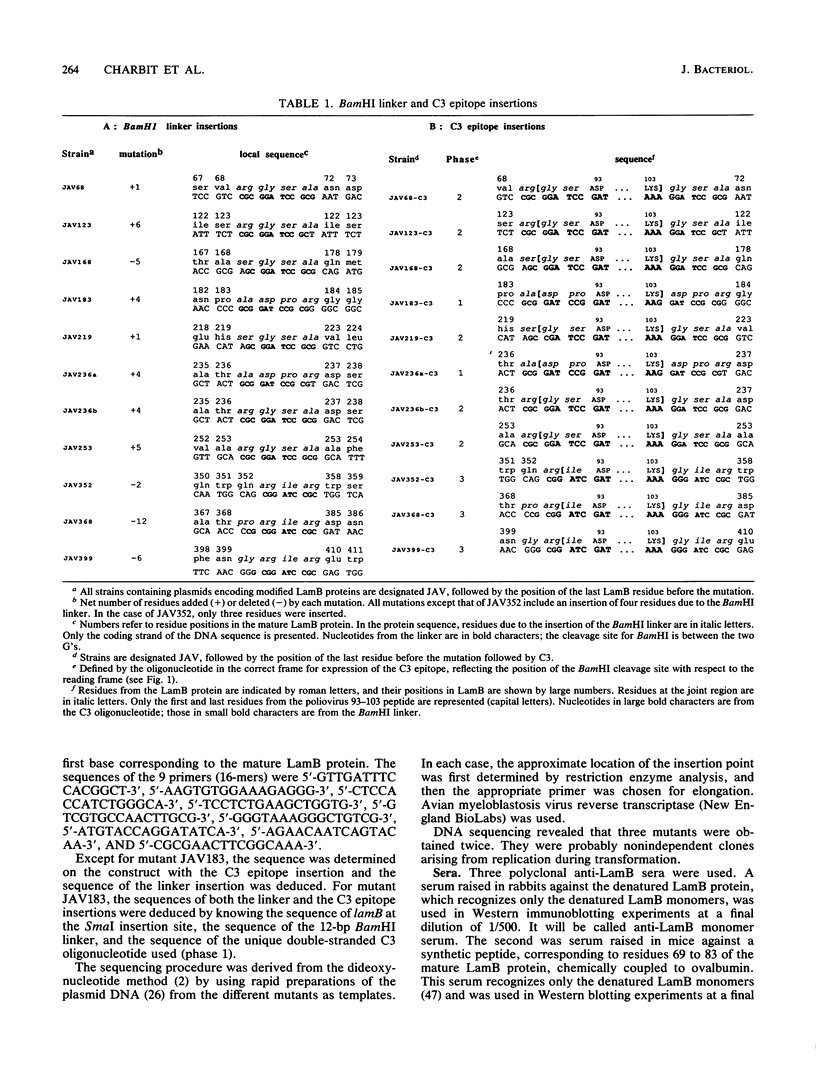
- Bouges-Bocquet B., Villarroya H., Hofnung M. Linker mutagenesis in the gene of an outer membrane protein of Escherichia coli, lamB. J Cell Biochem. 1984;24(3):217–228. doi: 10.1002/jcb.240240304. [DOI] [PubMed] [Google Scholar]
- Boulain J. C., Charbit A., Hofnung M. Mutagenesis by random linker insertion into the lamB gene of Escherichia coli K12. Mol Gen Genet. 1986 Nov;205(2):339–348. doi: 10.1007/BF00430448. [DOI] [PubMed] [Google Scholar]
- Braun-Breton C., Hofnung M. In vivo and in vitro functional alterations of the bacteriophage lambda receptor in lamB missense mutants of Escherichia coli K-12. J Bacteriol. 1981 Dec;148(3):845–852. doi: 10.1128/jb.148.3.845-852.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun-Breton C. Screening for lamB missense mutations which alter all lambda receptor activities in Escherichia coli K12. Ann Microbiol (Paris) 1984 Mar-Apr;135A(2):181–190. doi: 10.1016/s0769-2609(84)80001-0. [DOI] [PubMed] [Google Scholar]
- Broome-Smith J. K., Spratt B. G. A vector for the construction of translational fusions to TEM beta-lactamase and the analysis of protein export signals and membrane protein topology. Gene. 1986;49(3):341–349. doi: 10.1016/0378-1119(86)90370-7. [DOI] [PubMed] [Google Scholar]
- Charbit A., Boulain J. C., Ryter A., Hofnung M. Probing the topology of a bacterial membrane protein by genetic insertion of a foreign epitope; expression at the cell surface. EMBO J. 1986 Nov;5(11):3029–3037. doi: 10.1002/j.1460-2075.1986.tb04602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbit A., Clement J. M., Hofnung M. Further sequence analysis of the phage lambda receptor site. Possible implications for the organization of the lamB protein in Escherichia coli K12. J Mol Biol. 1984 May 25;175(3):395–401. doi: 10.1016/0022-2836(84)90355-3. [DOI] [PubMed] [Google Scholar]
- Charbit A., Gehring K., Nikaido H., Ferenci T., Hofnung M. Maltose transport and starch binding in phage-resistant point mutants of maltoporin. Functional and topological implications. J Mol Biol. 1988 Jun 5;201(3):487–496. doi: 10.1016/0022-2836(88)90630-4. [DOI] [PubMed] [Google Scholar]
- Charbit A., Hofnung M. Isolation of different bacteriophages using the LamB protein for adsorption on Escherichia coli K-12. J Virol. 1985 Feb;53(2):667–671. doi: 10.1128/jvi.53.2.667-671.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbit A., Molla A., Ronco J., Clément J. M., Favier V., Bahraoui E. M., Montagnier L., Leguern A., Hofnung M. Immunogenicity and antigenicity of conserved peptides from the envelope of HIV-1 expressed at the surface of recombinant bacteria. AIDS. 1990 Jun;4(6):545–551. doi: 10.1097/00002030-199006000-00008. [DOI] [PubMed] [Google Scholar]
- Clément J. M., Hofnung M. Gene sequence of the lambda receptor, an outer membrane protein of E. coli K12. Cell. 1981 Dec;27(3 Pt 2):507–514. doi: 10.1016/0092-8674(81)90392-5. [DOI] [PubMed] [Google Scholar]
- Desaymard C., Débarbouillé M., Jolit M., Schwartz M. Mutations affecting antigenic determinants of an outer membrane protein of Escherichia coli. EMBO J. 1986 Jun;5(6):1383–1388. doi: 10.1002/j.1460-2075.1986.tb04371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplay P., Szmelcman S., Bedouelle H., Hofnung M. Silent and functional changes in the periplasmic maltose-binding protein of Escherichia coli K12. I. Transport of maltose. J Mol Biol. 1987 Apr 20;194(4):663–673. doi: 10.1016/0022-2836(87)90243-9. [DOI] [PubMed] [Google Scholar]
- Freimuth P. I., Taylor J. W., Kaiser E. T. Introduction of guest peptides into Escherichia coli alkaline phosphatase. Excision and purification of a dynorphin analogue from an active chimeric protein. J Biol Chem. 1990 Jan 15;265(2):896–901. [PubMed] [Google Scholar]
- Gabay J., Benson S., Schwartz M. Genetic mapping of antigenic determinants on a membrane protein. J Biol Chem. 1983 Feb 25;258(4):2410–2414. [PubMed] [Google Scholar]
- Gabay J., Yasunaka K. Interaction of the lamB protein with the peptidoglycan layer in Escherichia coli K12. Eur J Biochem. 1980 Feb;104(1):13–18. doi: 10.1111/j.1432-1033.1980.tb04393.x. [DOI] [PubMed] [Google Scholar]
- Gehring K., Charbit A., Brissaud E., Hofnung M. Bacteriophage lambda receptor site on the Escherichia coli K-12 LamB protein. J Bacteriol. 1987 May;169(5):2103–2106. doi: 10.1128/jb.169.5.2103-2106.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., So M., McCarthy B. J. In vitro mutagenesis of a circular DNA molecule by using synthetic restriction sites. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6012–6016. doi: 10.1073/pnas.75.12.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine H. G., Francis G., Lee K. S., Ferenci T. Genetic analysis of sequences in maltoporin that contribute to binding domains and pore structure. J Bacteriol. 1988 Apr;170(4):1730–1738. doi: 10.1128/jb.170.4.1730-1738.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horaud F., Crainic R., Van der Werf S., Blondel B., Wichowski C., Akacem O., Bruneau P., Couillin P., Siffert O., Girard M. Identification and characterization of a continuous neutralization epitope (C3) present on type 1 poliovirus. Prog Med Virol. 1987;34:129–155. [PubMed] [Google Scholar]
- Lathe R., Kieny M. P., Skory S., Lecocq J. P. Linker tailing: unphosphorylated linker oligonucleotides for joining DNA termini. DNA. 1984;3(2):173–182. doi: 10.1089/dna.1984.3.173. [DOI] [PubMed] [Google Scholar]
- LeClerc C., Martineau P., Van der Werf S., Deriaud E., Duplay P., Hofnung M. Induction of virus-neutralizing antibodies by bacteria expressing the C3 poliovirus epitope in the periplasm. The route of immunization influences the isotypic distribution and the biologic activity of the antipoliovirus antibodies. J Immunol. 1990 Apr 15;144(8):3174–3182. [PubMed] [Google Scholar]
- Manoil C. Analysis of protein localization by use of gene fusions with complementary properties. J Bacteriol. 1990 Feb;172(2):1035–1042. doi: 10.1128/jb.172.2.1035-1042.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. A genetic approach to analyzing membrane protein topology. Science. 1986 Sep 26;233(4771):1403–1408. doi: 10.1126/science.3529391. [DOI] [PubMed] [Google Scholar]
- Manoil C., Mekalanos J. J., Beckwith J. Alkaline phosphatase fusions: sensors of subcellular location. J Bacteriol. 1990 Feb;172(2):515–518. doi: 10.1128/jb.172.2.515-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla A., Charbit A., Le Guern A., Ryter A., Hofnung M. Antibodies against synthetic peptides and the topology of LamB, an outer membrane protein from Escherichia coli K12. Biochemistry. 1989 Oct 3;28(20):8234–8241. doi: 10.1021/bi00446a040. [DOI] [PubMed] [Google Scholar]
- Murphy C. K., Kalve V. I., Klebba P. E. Surface topology of the Escherichia coli K-12 ferric enterobactin receptor. J Bacteriol. 1990 May;172(5):2736–2746. doi: 10.1128/jb.172.5.2736-2746.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T., Ishii J. N. Molecular weights and subunit structure of LamB proteins. Ann Microbiol (Paris) 1982 Jan;133A(1):21–25. [PubMed] [Google Scholar]
- Neuhaus J. M. The receptor protein of phage lambda: purification, characterization and preliminary electrical studies in planar lipid bilayers. Ann Microbiol (Paris) 1982 Jan;133A(1):27–32. [PubMed] [Google Scholar]
- Newton S. M., Jacob C. O., Stocker B. A. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science. 1989 Apr 7;244(4900):70–72. doi: 10.1126/science.2468182. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Reid J., Fung H., Gehring K., Klebba P. E., Nikaido H. Targeting of porin to the outer membrane of Escherichia coli. Rate of trimer assembly and identification of a dimer intermediate. J Biol Chem. 1988 Jun 5;263(16):7753–7759. [PubMed] [Google Scholar]
- Rodseth L. E., Martineau P., Duplay P., Hofnung M., Quiocho F. A. Crystallization of genetically engineered active maltose-binding proteins, including an immunogenic viral epitope insertion. J Mol Biol. 1990 Jun 20;213(4):607–611. doi: 10.1016/S0022-2836(05)80246-3. [DOI] [PubMed] [Google Scholar]
- Ronco J., Charbit A., Hofnung M. Creation of targets for proteolytic cleavage in the LamB protein of E coli K12 by genetic insertion of foreign sequences: implications for topological studies. Biochimie. 1990 Feb-Mar;72(2-3):183–189. doi: 10.1016/0300-9084(90)90144-6. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Tsugita A., Schwartz M., Rosenbusch J. P. Topology of phage lambda receptor protein. Mapping targets of proteolytic cleavage in relation to binding sites for phage or monoclonal antibodies. J Biol Chem. 1984 Jun 25;259(12):7570–7576. [PubMed] [Google Scholar]
- Sen K., Nikaido H. In vitro trimerization of OmpF porin secreted by spheroplasts of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jan;87(2):743–747. doi: 10.1073/pnas.87.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmelcman S., Clément J. M., Jehanno M., Schwartz O., Montagnier L., Hofnung M. Export and one-step purification from Escherichia coli of a MalE-CD4 hybrid protein that neutralizes HIV in vitro. J Acquir Immune Defic Syndr. 1990;3(9):859–872. [PubMed] [Google Scholar]
- Vos-Scheperkeuter G. H., Witholt B. Assembly pathway of newly synthesized LamB protein an outer membrane protein of Escherichia coli K-12. J Mol Biol. 1984 Jun 5;175(4):511–528. doi: 10.1016/0022-2836(84)90182-7. [DOI] [PubMed] [Google Scholar]
- van der Werf S., Charbit A., Leclerc C., Mimic V., Ronco J., Girard M., Hofnung M. Critical role of neighbouring sequences on the immunogenicity of the C3 poliovirus neutralization epitope expressed at the surface of recombinant bacteria. Vaccine. 1990 Jun;8(3):269–277. doi: 10.1016/0264-410x(90)90057-s. [DOI] [PubMed] [Google Scholar]
- van der Werf S., Wychowski C., Bruneau P., Blondel B., Crainic R., Horodniceanu F., Girard M. Localization of a poliovirus type 1 neutralization epitope in viral capsid polypeptide VP1. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5080–5084. doi: 10.1073/pnas.80.16.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]




