Abstract
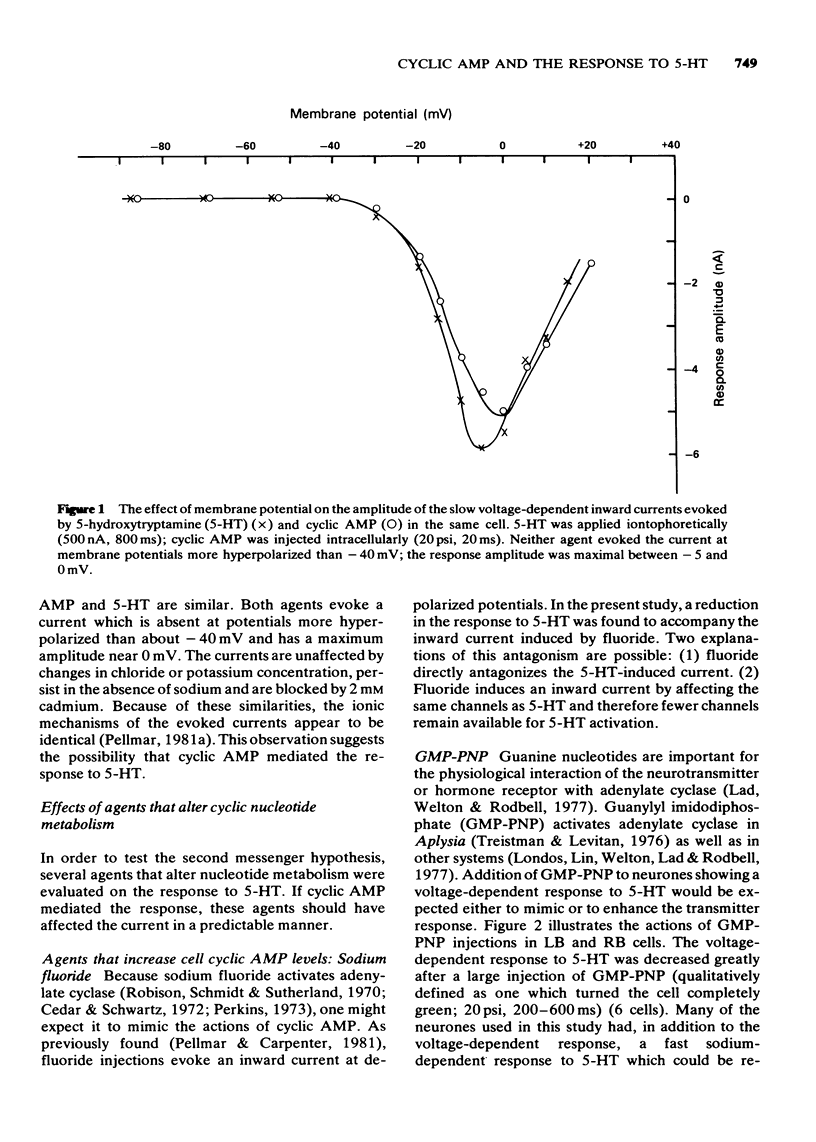
1 The possibility that cyclic adenosine 3',5'-monophosphate (cyclic AMP) mediates a voltage-dependent inward current elicited by 5-hydroxytryptamine (5-HT) in RB and LB cells of the abdominal ganglion of Aplysia was tested. 2 Intracellular injection of cyclic AMP elicited an inward current with a similar time course, potential dependence and ionic sensitivity as the response to 5-HT. 3 Intracellular injection of guanylyl imidodiphosphate (GMP-PNP), which activated adenylate cyclase, neither mimicked nor enhanced the 5-HT-evoked current. On the contrary, it reduced the current. 4 The phosphodiesterase inhibitors, Ro20-1724, isobutyl methylxanthine (IBMX) and theophylline, each antagonized the voltage-dependent response to 5-HT. To varying degrees they each induced an inward current. 5 The adenylate cyclase antagonist, dithiobisnitrobenzoic acic (DTNB), had no effect on the response to 5-HT when applied either intracellularly or extracellularly. Intracellular injection of the phosphodiesterase activator imidazole also had no effect. 6 Tubocurarine and neostigmine did not reduce the voltage-dependent inward current evoked by 5-HT; methysergide elicited an inward current. 7 Although the observations that cyclic AMP and 5-HT can evoke similar voltage-dependent inward currents in RB and LB neurones of Aplysia might suggest a second messenger role for the cyclic nucleotide, the pharmacological data are inconsistent with this hypothesis.
Full text
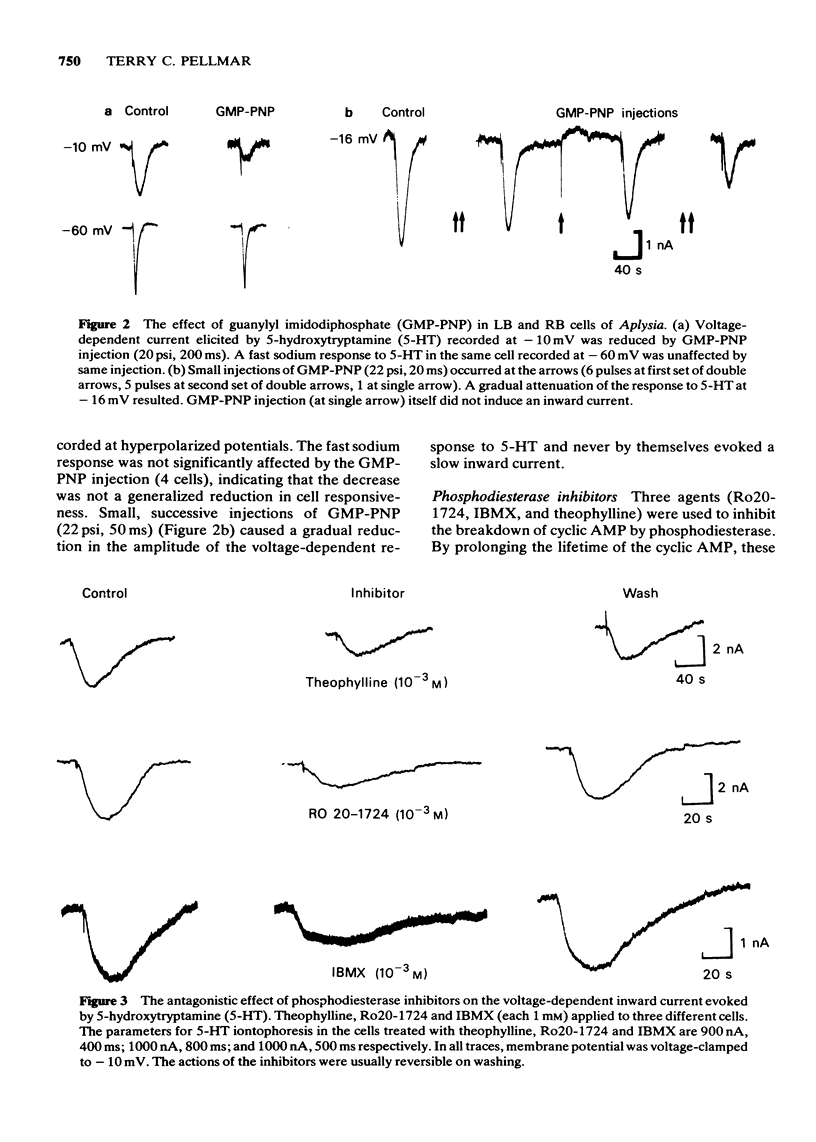
PDF

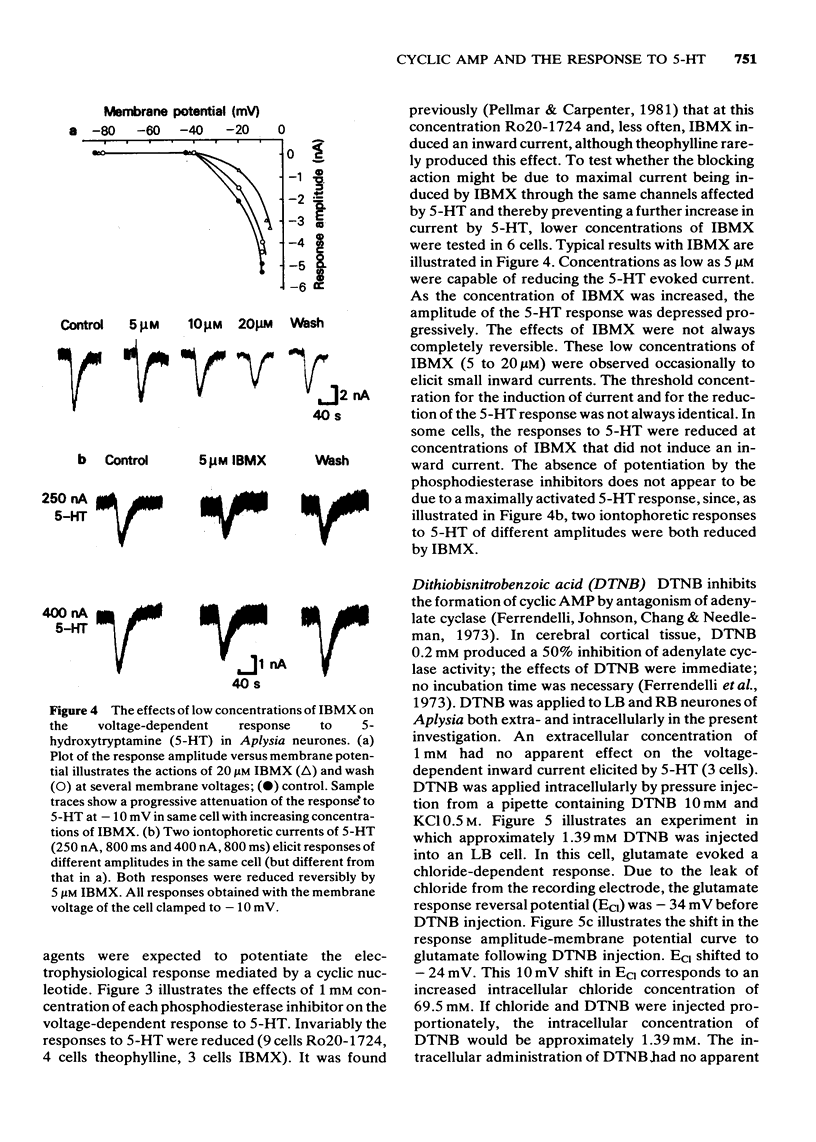
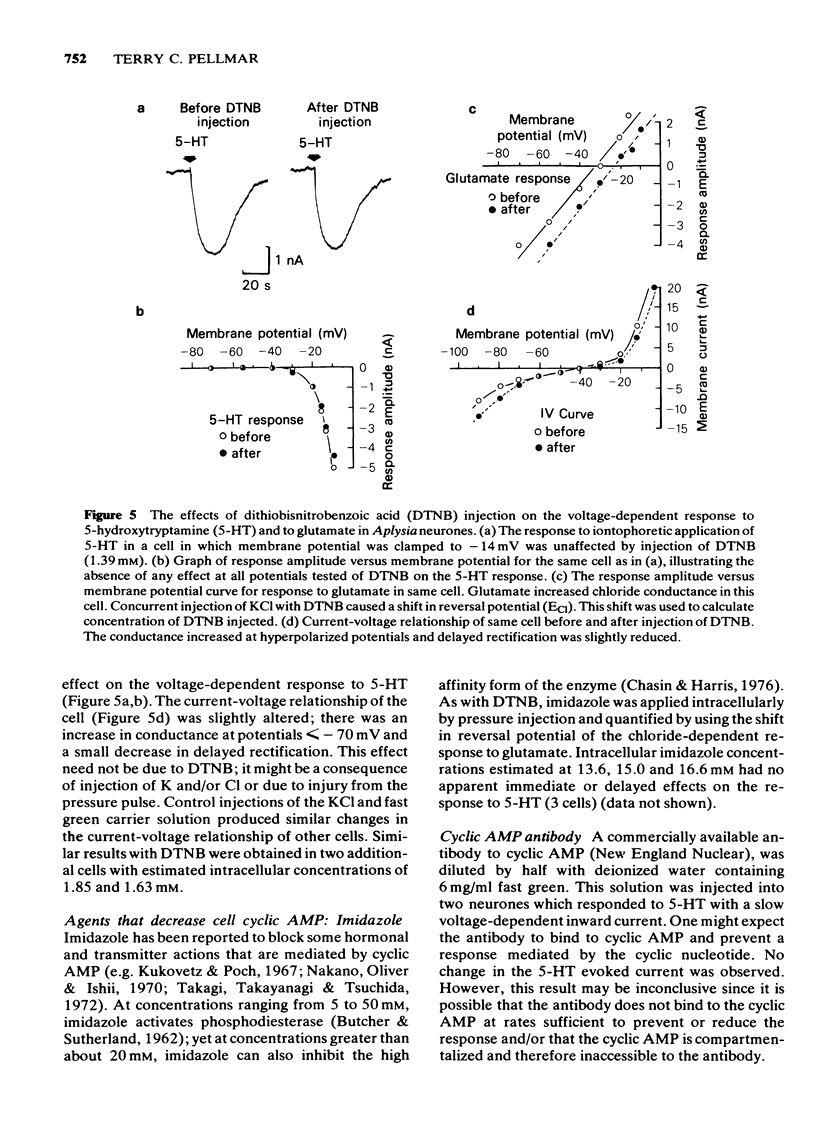




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Beam K. G., Greengard P. Cyclic nucleotides, protein phosphorylation and synaptic function. Cold Spring Harb Symp Quant Biol. 1976;40:157–168. doi: 10.1101/sqb.1976.040.01.017. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Caulfield M. P., Kirby P. J. Relation between catecholamine-induced cyclic AMP changes and hyperpolarization in isolated rat sympathetic ganglia. J Physiol. 1979 May;290(2):441–451. doi: 10.1113/jphysiol.1979.sp012782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. Cyclic adenosine monophosphate in the nervous system of Aplysia californica. II. Effect of serotonin and dopamine. J Gen Physiol. 1972 Nov;60(5):570–587. doi: 10.1085/jgp.60.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasin M., Harris D. N. Inhibitory and activators of cyclic nucleotide phosphodiesterase. Adv Cyclic Nucleotide Res. 1976;7:225–264. [PubMed] [Google Scholar]
- Deterre P., Paupardin-Tritsch D., Bockaert J., Gerschenfeld H. M. Role of cyclic AMP in a serotonin-evoked slow inward current in snail neurones. Nature. 1981 Apr 30;290(5809):783–785. doi: 10.1038/290783a0. [DOI] [PubMed] [Google Scholar]
- Drummond A. H., Benson J. A., Levitan I. B. Serotonin-induced hyperpolarization of an indentified Aplysia neuron is mediated by cyclic AMP. Proc Natl Acad Sci U S A. 1980 Aug;77(8):5013–5017. doi: 10.1073/pnas.77.8.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrendelli J. A., Johnson E. M., Jr, Chang M., Needleman P. Inhibition of brain adenylate cyclase by ethacrynic acid and dithiobisnitrobenzoic acid. Biochem Pharmacol. 1973 Dec 1;22(23):3133–3136. doi: 10.1016/0006-2952(73)90206-2. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M., Paupardin-Tritsch D. Ionic mechanisms and receptor properties underlying the responses of molluscan neurones to 5-hydroxytryptamine. J Physiol. 1974 Dec;243(2):427–456. doi: 10.1113/jphysiol.1974.sp010761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C. Adenosine receptors in frog sinus venosus: slow inhibitory potentials produced by adenine compounds and acetylcholine. J Physiol. 1979 Aug;293:23–49. doi: 10.1113/jphysiol.1979.sp012877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C. Presynaptic function in Helix pomatia is changed by phosphodiesterase inhibitors, cyclic nucleotide derivatives, and neurotransmitter induced cAMP. Comp Biochem Physiol C. 1981;68C(1):21–27. doi: 10.1016/0306-4492(81)90032-0. [DOI] [PubMed] [Google Scholar]
- Kukovetz W. R., Pöch G. The action of imidazole on the effects of methyl-xanthines and catecholamines on cardiac contraction and phosphorylase activity. J Pharmacol Exp Ther. 1967 Jun;156(3):514–521. [PubMed] [Google Scholar]
- Lad P. M., Welton A. F., Rodbell M. Evidence for distinct guanine nucleotide sites in the regulation of the glucagon receptor and of adenylate cyclase activity. J Biol Chem. 1977 Sep 10;252(17):5942–5946. [PubMed] [Google Scholar]
- Levitan I. B., Madsen C. J., Barondes S. H. Cyclic AMP and amine effects on phosphorylation of specific protein in abdominal ganglion of Aplysia californica; localization and kinetic analysis. J Neurobiol. 1974;5(6):511–525. doi: 10.1002/neu.480050604. [DOI] [PubMed] [Google Scholar]
- Londos C., Lin M. C., Welton A. F., Lad P. M., Rodbell M. Reversible activation of hepatic adenylate cyclase by guanyl-5'-yl-(alpha,beta-methylene)diphosphonate and guanyl-5'-yl imidodiphosphate. J Biol Chem. 1977 Aug 10;252(15):5180–5182. [PubMed] [Google Scholar]
- MacDermot J., Higashida H., Wilson S. P., Matsuzawa H., Minna J., Nirenberg M. Adenylate cyclase and acetylcholine release regulated by separate serotonin receptors of somatic cell hybrids. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1135–1139. doi: 10.1073/pnas.76.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano J., Oliver R., Ishii T. Effect of imidazole and its derivatives on norepinephrine-, ACTH-, theophylline- and dibutyryl cyclic AMP-induced lipolysis in isolated rat fat cells. Pharmacology. 1970;3(5):273–281. doi: 10.1159/000136081. [DOI] [PubMed] [Google Scholar]
- Pellmar T. C., Carpenter D. O. Cyclic AMP induces a voltage-dependent current in neurones of Aplysia californica. Neurosci Lett. 1981 Mar 10;22(2):151–157. doi: 10.1016/0304-3940(81)90079-3. [DOI] [PubMed] [Google Scholar]
- Pellmar T. C., Carpenter D. O. Serotonin induces a voltage-sensitive calcium current in neurons of Aplysia californica. J Neurophysiol. 1980 Sep;44(3):423–439. doi: 10.1152/jn.1980.44.3.423. [DOI] [PubMed] [Google Scholar]
- Pellmar T. C. Ionic mechanism of a voltage-dependent current elicited by cyclic AMP. Cell Mol Neurobiol. 1981 Mar;1(1):87–97. doi: 10.1007/BF00736041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins J. P. Adenyl cyclase. Adv Cyclic Nucleotide Res. 1973;3:1–64. [PubMed] [Google Scholar]
- Robison G. A., Schmidt M. J., Sutherland E. W. On the development and properties of the brain adenyl cyclase system. Adv Biochem Psychopharmacol. 1970;3:11–30. [PubMed] [Google Scholar]
- Takagi K., Takayanagi I., Tsuchida Y. The effects of caffeine and imidazole on the actions of beta- and alpha-adrenergic stimulants, papaverine and cyclic 3', 5'-AMP. Jpn J Pharmacol. 1972 Jun;22(3):403–409. doi: 10.1254/jjp.22.403. [DOI] [PubMed] [Google Scholar]
- Treistman S. N., Drake P. F. The effects of cyclic nucleotide agents on neurons in Aplysia. Brain Res. 1979 Jun 8;168(3):643–647. doi: 10.1016/0006-8993(79)90321-4. [DOI] [PubMed] [Google Scholar]
- Treistman S. N., Levitan I. B. Intraneuronal guanylyl-imidodiphosphate injection mimics long-term synaptic hyperpolarization in Aplysia. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4689–4692. doi: 10.1073/pnas.73.12.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W. A., Goldner M. M. Voltage clamping with a single microelectrode. J Neurobiol. 1975 Jul;6(4):411–422. doi: 10.1002/neu.480060406. [DOI] [PubMed] [Google Scholar]


