Abstract
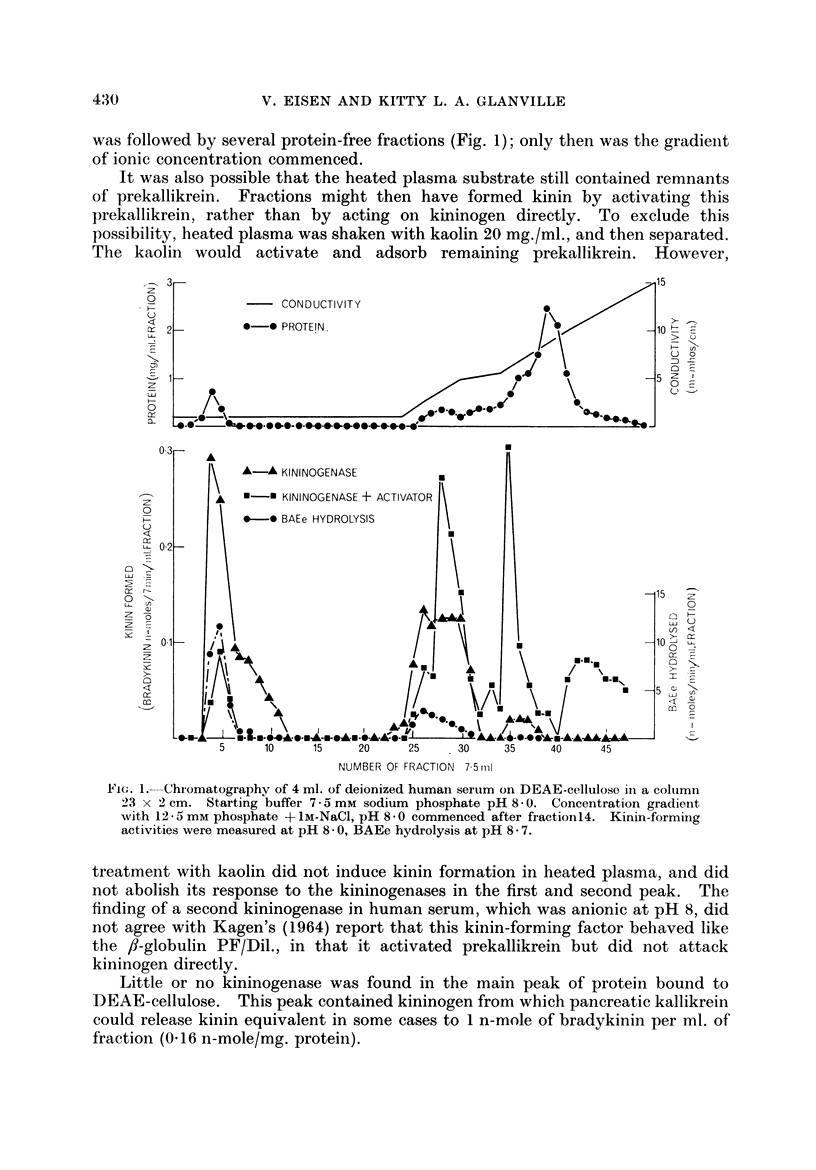
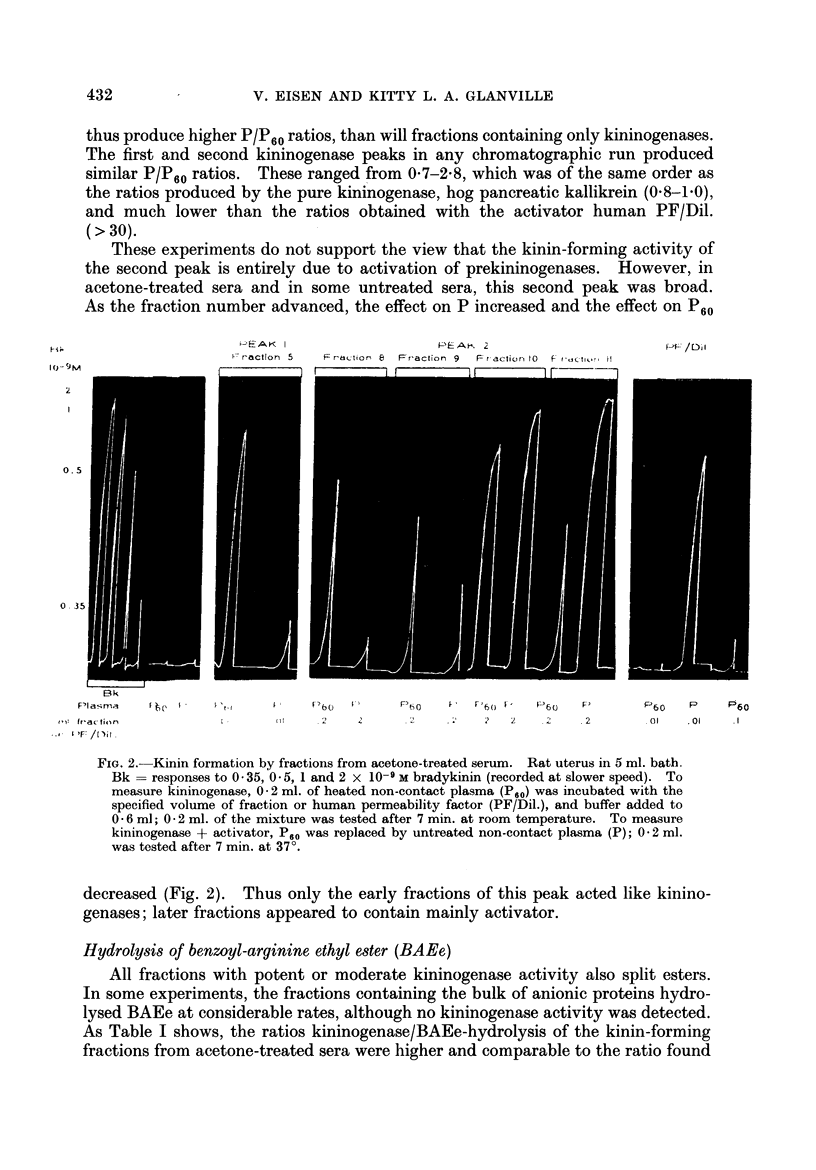
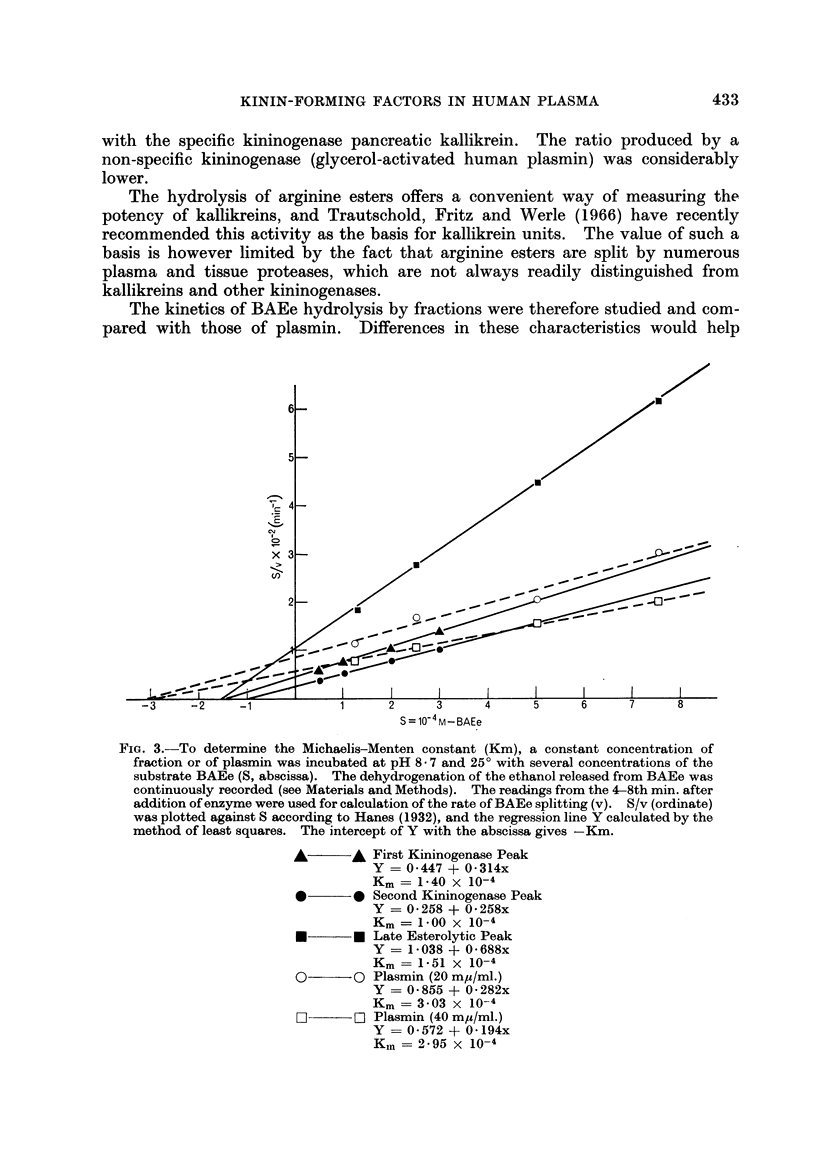
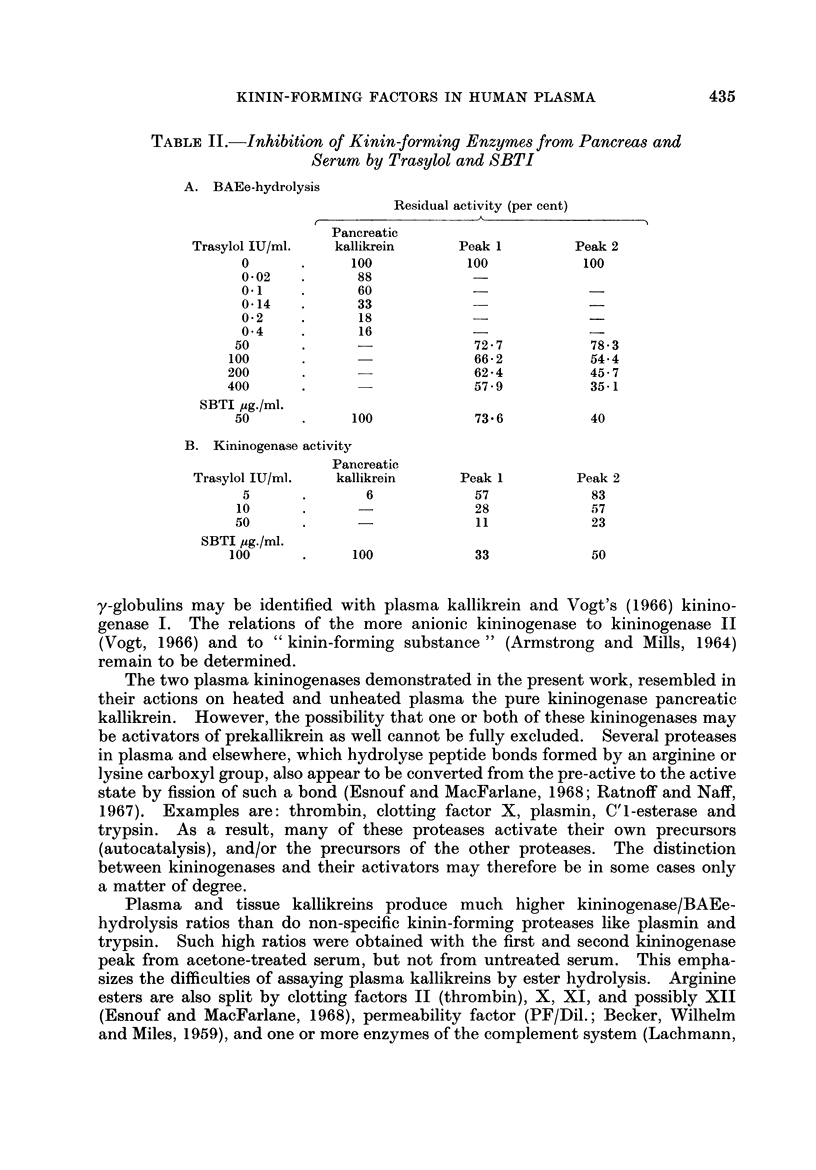
The kinin-forming proteases in human serum were studied. Two serum kallikreins, i.e. enzymes acting directly and specifically on kinin-yielding globulin (kininogen), were separated by ion-exchange chromatography. They were differentiated from plasmin by their high ratio kinin-forming/esterolytic activity, by the absence of fibrinolytic activity, and by a lower Michaelis-Menten constant for the hydrolysis of an arginine ester. Their relationship to enzymes which induce kinin formation mainly by activating serum prekallikreins, was studied.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG D. A., MILLS G. L. A HIGHLY PURIFIED KININ-FORMING ENZYME FROM HUMAN PLASMA. Biochem Pharmacol. 1964 Oct;13:1393–1402. doi: 10.1016/0006-2952(64)90188-1. [DOI] [PubMed] [Google Scholar]
- ASTRUP T., MULLERTZ S. The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys. 1952 Oct;40(2):346–351. doi: 10.1016/0003-9861(52)90121-5. [DOI] [PubMed] [Google Scholar]
- BECKER E. L., WILHELM D. L., MILES A. A. Enzymic nature of the serum globulin permeability factor. Nature. 1959 May 2;183(4670):1264–1265. doi: 10.1038/1831264a0. [DOI] [PubMed] [Google Scholar]
- Eisen V. Observations on intrinsic kinin-forming factors in human plasma: the effect of acid, acetone, chloroform, heat and euglobulin separation on kinin formation. J Physiol. 1963 May;166(3):496–513. doi: 10.1113/jphysiol.1963.sp007119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esnouf M. P., Macfarlane R. G. Enzymology and the blood clotting mechanism. Adv Enzymol Relat Areas Mol Biol. 1968;30:255–315. doi: 10.1002/9780470122754.ch6. [DOI] [PubMed] [Google Scholar]
- Hanes C. S. Studies on plant amylases: The effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem J. 1932;26(5):1406–1421. doi: 10.1042/bj0261406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAGEN L. J., LEDDY J. P., BECKER E. L. THE PRESENCE OF TWO PERMEABILITY GLOBULINS IN HUMAN SERUM. J Clin Invest. 1963 Aug;42:1353–1361. doi: 10.1172/JCI104819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAGEN L. J. SOME BIOCHEMICAL AND PHYSICAL PROPERTIES OF THE HUMAN PERMEABILITY GLOBULINS. Br J Exp Pathol. 1964 Dec;45:604–611. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILES A. A., MILES E. M. Vascular reactions to histamine, histamine-liberator and leukotaxine in the skin of guinea-pigs. J Physiol. 1952 Oct;118(2):228–257. doi: 10.1113/jphysiol.1952.sp004789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya H., Yamazaki K., Fukushima H. Biochemical studies on kallikreins and their related substances. 3. Preliminary experiment of studies on human plasma kallikrein. J Biochem. 1965 Oct;58(4):315–319. doi: 10.1093/oxfordjournals.jbchem.a128207. [DOI] [PubMed] [Google Scholar]
- Ratnoff O. D., Naff G. B. The conversion of C'IS to C'1 esterase by plasmin and trypsin. J Exp Med. 1967 Feb 1;125(2):337–358. doi: 10.1084/jem.125.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWERT G. W., TAKENAKA Y. A spectrophotometric determination of trypsin and chymotrypsin. Biochim Biophys Acta. 1955 Apr;16(4):570–575. doi: 10.1016/0006-3002(55)90280-8. [DOI] [PubMed] [Google Scholar]
- WEBSTER M. E., PIERCE J. V. Action of the kallikreins on synthetic ester substrates. Proc Soc Exp Biol Med. 1961 May;107:186–191. doi: 10.3181/00379727-107-26575. [DOI] [PubMed] [Google Scholar]
- Zeitlin I. J., Smith A. N. 5-hydroxyindoles and kinins in the carcinoid and dumping syndromes. Lancet. 1966 Nov 5;2(7471):986–991. doi: 10.1016/s0140-6736(66)92924-2. [DOI] [PubMed] [Google Scholar]


