Abstract
The effect of α- and β-amanitin on mouse kidney and rat kidney have been studied.
The experiments on adult male mice show that (1) an MLD of α- or β-amanitin always produces necrosis in the kidneys but never in the liver; (2) with the doses used (up to 3 MLD) necrosis of the kidneys never appears less than 3 days after injection; (3) necrosis of the liver only appears with doses above MLD and generally within 2 days.
No lesions were found in rat kidney after injection of amanitin.
Mice injected with the conjugate of β-amanitin with albumin from rabbit serum all died from necrosis of the liver without any lesions in the kidney, 3 days after i.p. injection. It has been deduced that amanitin poisoning in mouse kidney depends on reabsorption in the tubules, that either the liver or the kidney can be the target organ of amanitin (depending on the dose) and that in rats nephrosis is prevented because there is no reabsorption of amanitin in the kidney tubules.
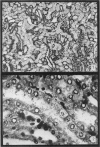
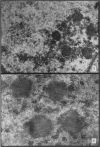
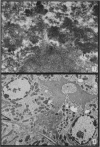
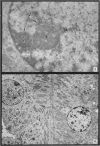
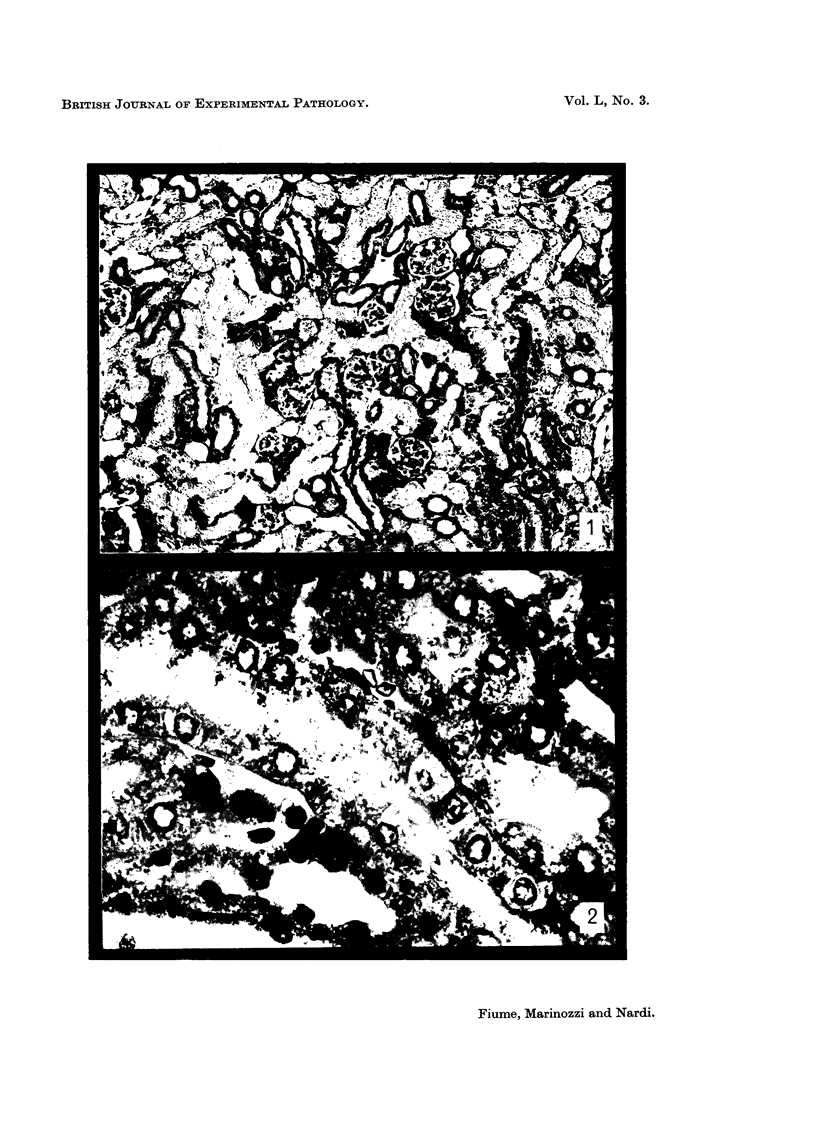
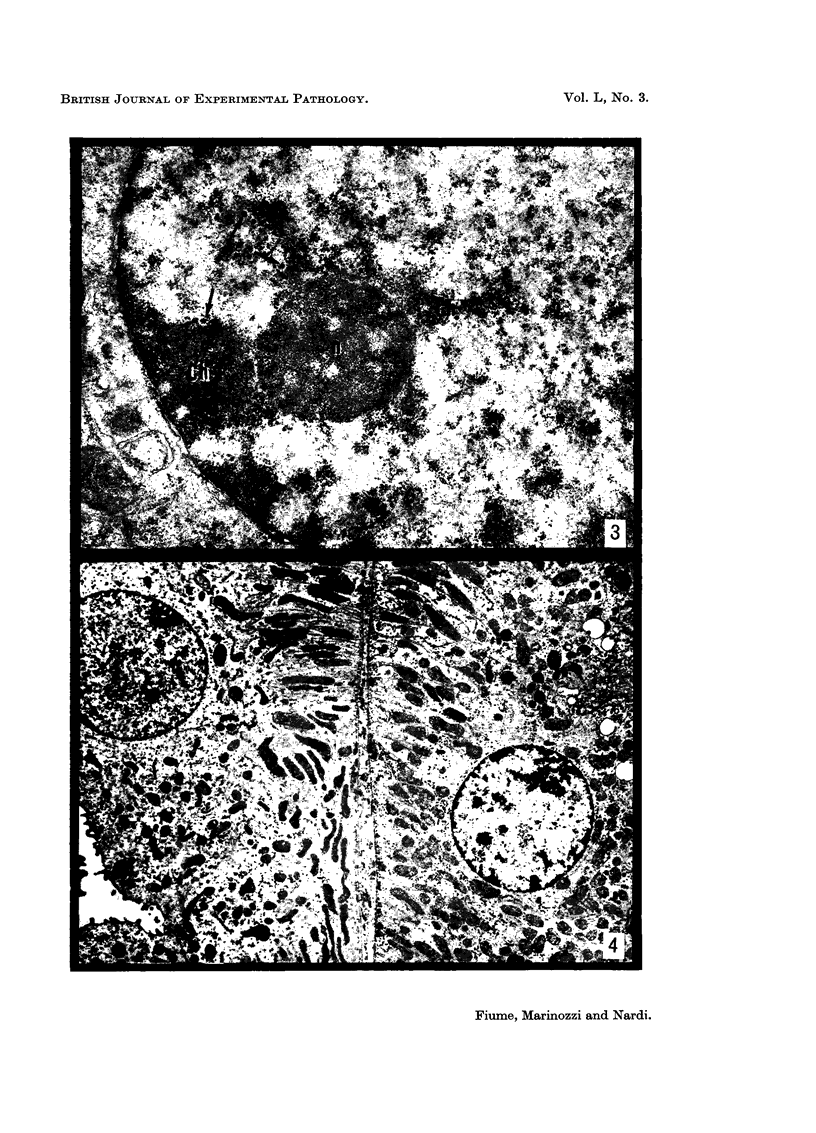
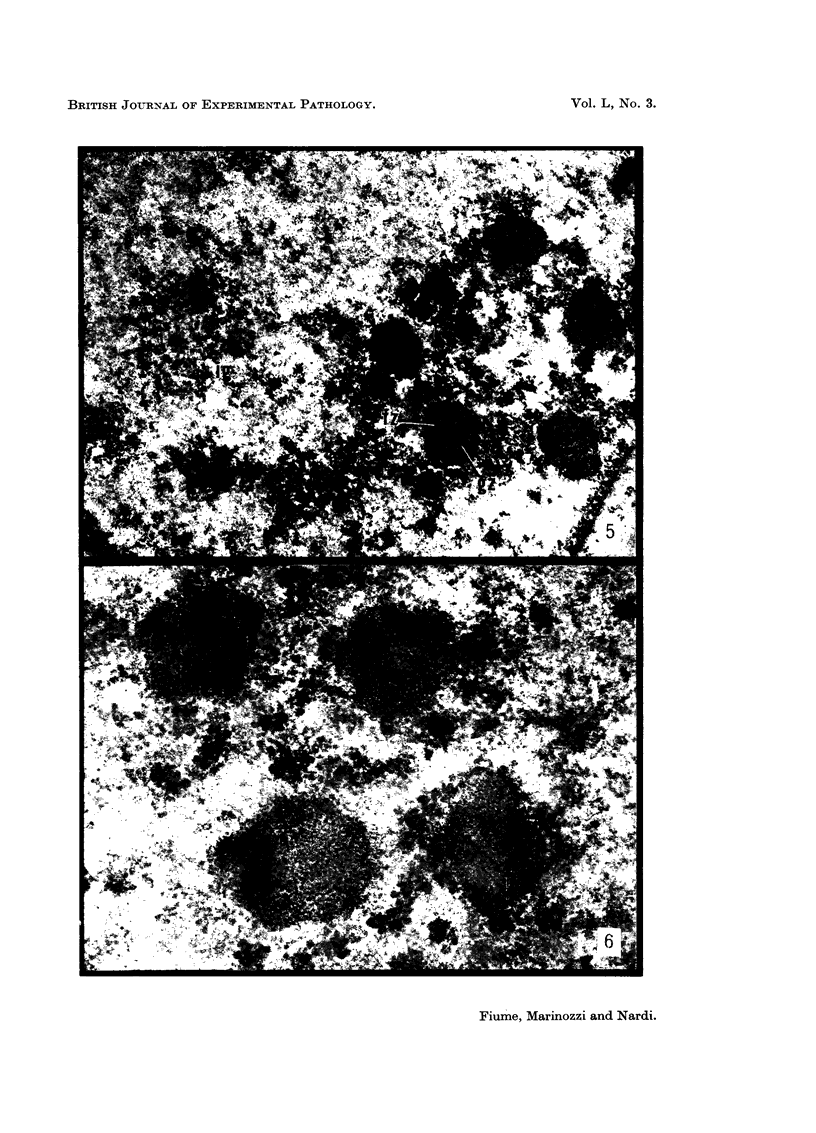
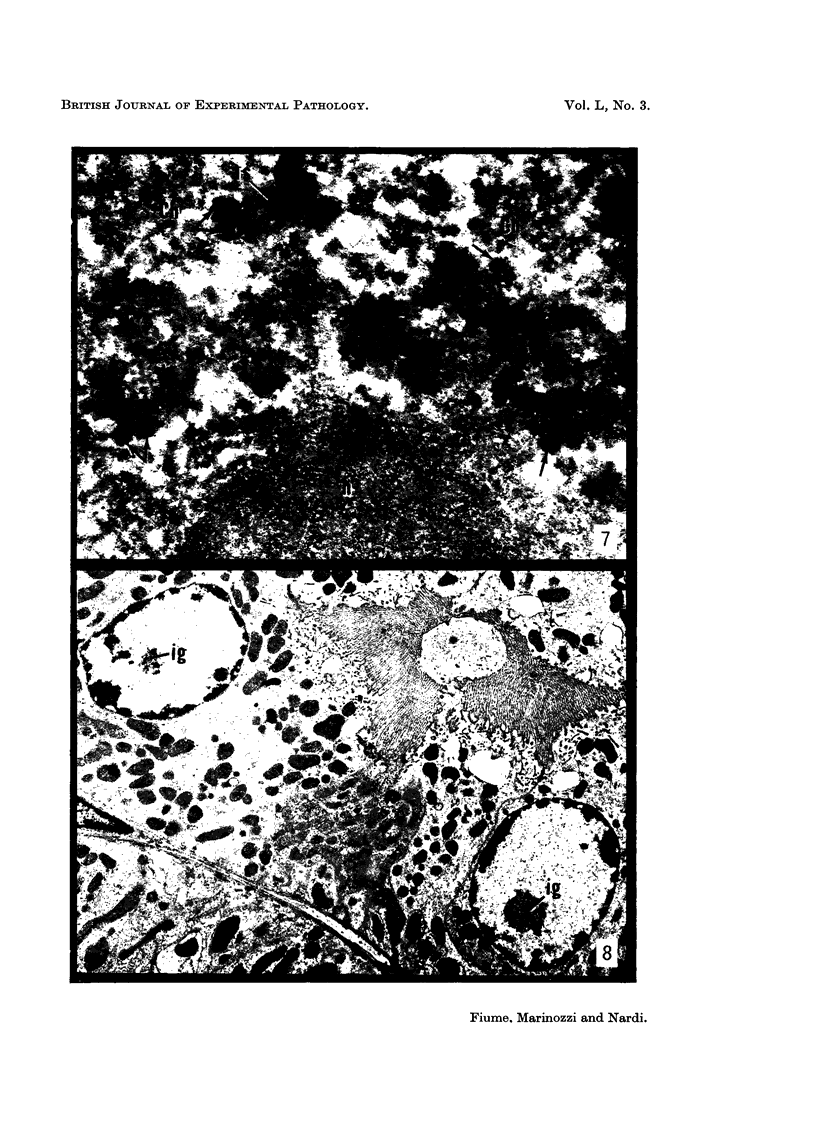
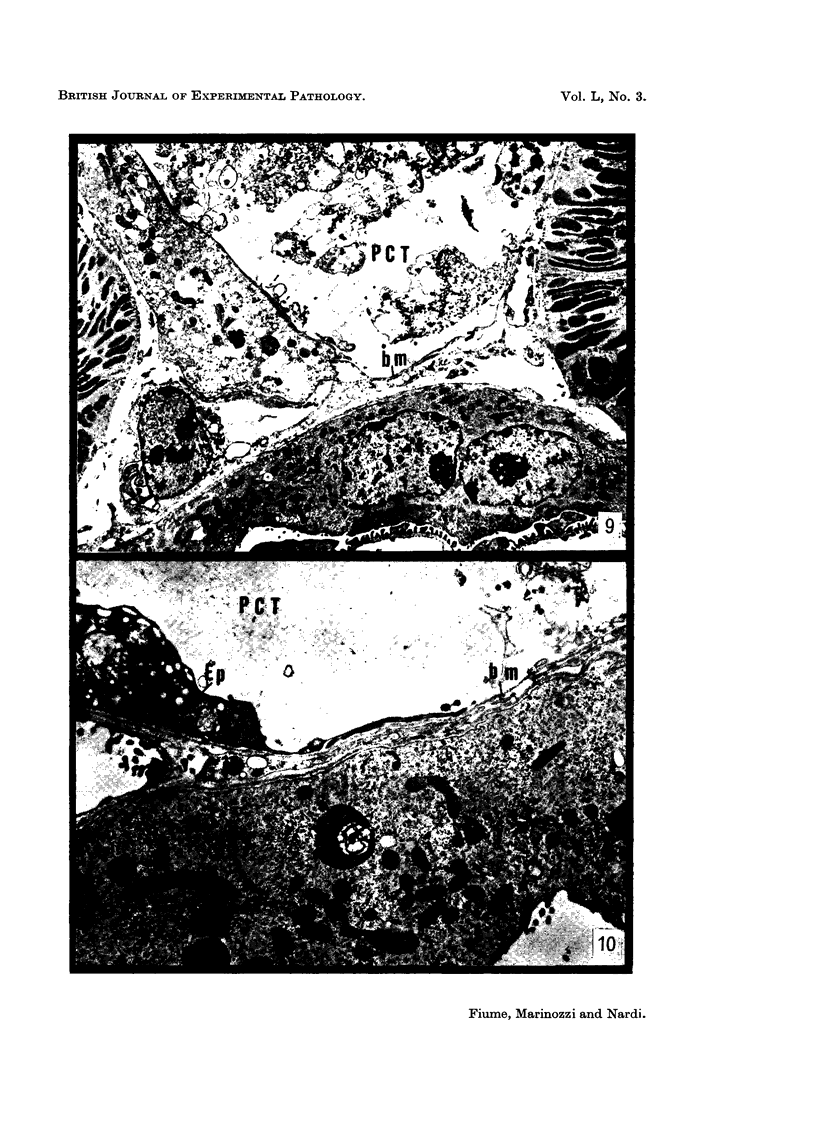
The earliest ultrastructural changes occur in nuclei. First there is fragmentation of nucleoli and segregation of its granular and fibrillar components. At a second stage these fragments fall in number and then tend to disappear. At the same time there is a temporary increase in the number of perichromatin granules. Chromatin condensates at the borders of the nucleus and there is a big increase in numbers of interchromatin granules at the centre.
Changes in the cytoplasm appear just before necrosis sets in.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fiume L., Laschi R. Lesioni ultrastrutturali prodotte nelle cellule parenchimali epatiche dalla falloidina e dalla alpha-amanitina. Sperimentale. 1965 Sep-Oct;115(5):288–297. [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- Permutt M. A., Parker C. W., Utiger R. D. Immunochemical studies with lysine vasopressin. Endocrinology. 1966 Apr;78(4):809–814. doi: 10.1210/endo-78-4-809. [DOI] [PubMed] [Google Scholar]
- SCHOEFL G. I. THE EFFECT OF ACTINOMYCIN D ON THE FINE STRUCTURE OF THE NUCLEOLUS. J Ultrastruct Res. 1964 Apr;10:224–243. doi: 10.1016/s0022-5320(64)80007-1. [DOI] [PubMed] [Google Scholar]
- SWIFT H. Studies on nuclear fine structure. Brookhaven Symp Biol. 1959 Nov;12:134–152. [PubMed] [Google Scholar]
- Simard R., Bernhard W. Le phénomène de la ségrégation nucléolaire: spécificité d'action de certains antimétabolites. Int J Cancer. 1966 Sep 15;1(5):463–479. doi: 10.1002/ijc.2910010506. [DOI] [PubMed] [Google Scholar]
- Stirpe F., Fiume L. Studies on the pathogenesis of liver necrosis by alpha-amanitin. Effect of alpha-amanitin on ribonucleic acid synthesis and on ribonucleic acid polymerase in mouse liver nuclei. Biochem J. 1967 Nov;105(2):779–782. doi: 10.1042/bj1050779. [DOI] [PMC free article] [PubMed] [Google Scholar]