Abstract
Mini-F plasmids cannot replicate in Escherichia coli strains (delta rpoH) lacking sigma 32, presumably because transcription of the repE gene encoding the replication initiator protein (RepE protein) depends mostly on RNA polymerase containing sigma 32. We have isolated and characterized mini-F mutants able to replicate in delta rpoH cells. Contrary to the initial expectation, five mutants with mutations in the repE coding region that produce altered RepE proteins were obtained. The mutations caused replacement of a single amino acid: the 92nd glutamic acid was replaced by lysine (repE10, repE16, and repE25) or glycine (repE22) or the 109th glutamic acid was replaced by lysine (repE26). These plasmids overproduced RepE protein and exhibited very high copy numbers. Two major activities of mutated RepE proteins have been determined in vivo; the autogenous repressor activity was significantly reduced, whereas the initiator activity was much enhanced in all mutants. These results indicate the importance of a small central region of RepE protein for both initiator and repressor activities. Thus the decreased repE transcription in delta rpoH cells can be compensated for by an increased initiator activity and a decreased repressor activity of RepE, resulting in the increased synthesis of hyperactive RepE protein.
Full text
PDF

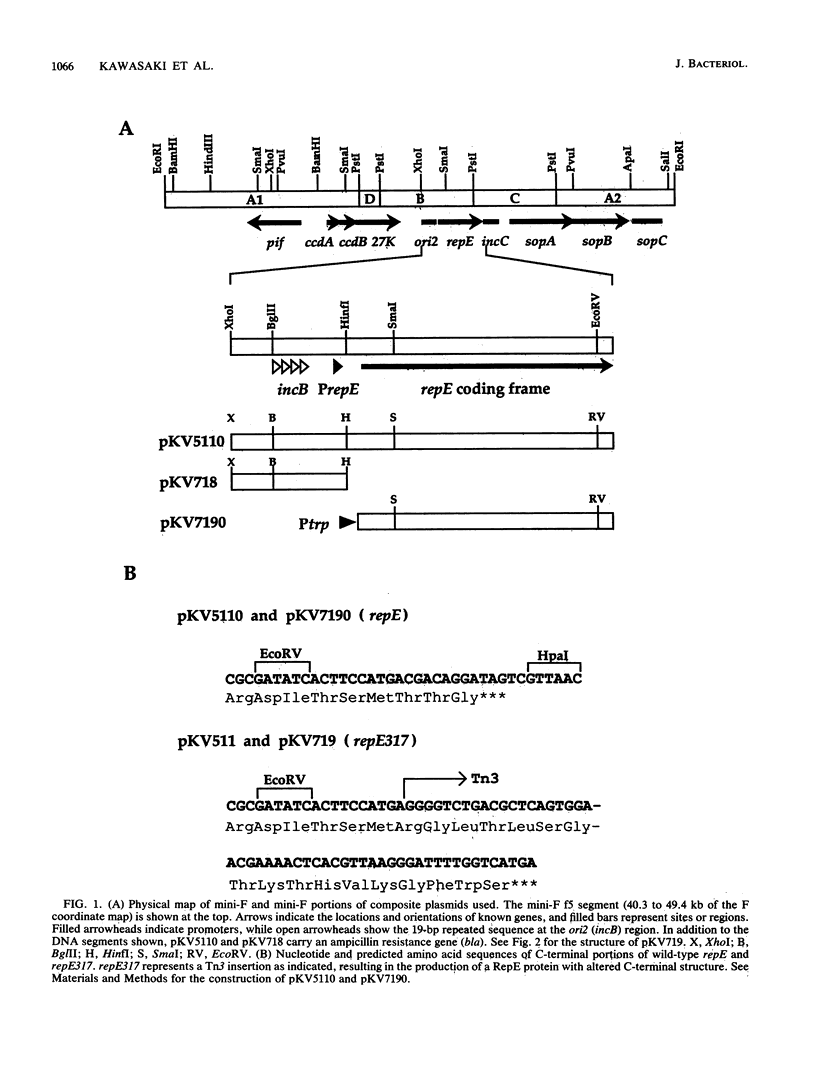






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong K. A., Acosta R., Ledner E., Machida Y., Pancotto M., McCormick M., Ohtsubo H., Ohtsubo E. A 37 X 10(3) molecular weight plasmid-encoded protein is required for replication and copy number control in the plasmid pSC101 and its temperature-sensitive derivative pHS1. J Mol Biol. 1984 May 25;175(3):331–348. doi: 10.1016/0022-2836(84)90352-8. [DOI] [PubMed] [Google Scholar]
- Bergquist P. L., Downard R. A., Caughey P. A., Gardner R. C., Lane H. E. Analysis of mini-F plasmid replication by transposition mutagenesis. J Bacteriol. 1981 Sep;147(3):888–899. doi: 10.1128/jb.147.3.888-899.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bex F., Piérard P., Desmyter A., Drèze P., Colet M., Couturier M. Mini-F E protein: the carboxy-terminal end is essential for E gene repression and mini-F copy number control. J Mol Biol. 1986 May 20;189(2):293–303. doi: 10.1016/0022-2836(86)90511-5. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnen G. E., Cox E. C. Conditional mutator gene in Escherichia coli: isolation, mapping, and effector studies. J Bacteriol. 1974 Feb;117(2):477–487. doi: 10.1128/jb.117.2.477-487.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich B. J., Scott J. R. A single amino acid difference between Rep proteins of P1 and P7 affects plasmid copy number. Plasmid. 1988 Mar;19(2):121–133. doi: 10.1016/0147-619x(88)90051-0. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Zurawski G., Yanofsky C. Structural and functional analysis of cloned deoxyribonucleic acid containing the trpR-thr region of the Escherichia coli chromosome. J Bacteriol. 1979 Oct;140(1):106–113. doi: 10.1128/jb.140.1.106-113.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsberg M., Ebbers J., Eichenlaub R. Mutations affecting replication and copy number control in plasmid mini-F both reside in the gene for the 29-kDa protein. Plasmid. 1985 Jul;14(1):53–63. doi: 10.1016/0147-619x(85)90032-0. [DOI] [PubMed] [Google Scholar]
- Hirano M., Shigesada K., Imai M. Construction and characterization of plasmid and lambda phage vector systems for study of transcriptional control in Escherichia coli. Gene. 1987;57(1):89–99. doi: 10.1016/0378-1119(87)90180-6. [DOI] [PubMed] [Google Scholar]
- Imber R. Regulation of expression of the cloned repE gene from the F plasmid of Escherichia coli. Gene. 1987;52(1):1–9. doi: 10.1016/0378-1119(87)90389-1. [DOI] [PubMed] [Google Scholar]
- Inuzuka M., Wada Y. A single amino acid alteration in the initiation protein is responsible for the DNA overproduction phenotype of copy number mutants of plasmid R6K. EMBO J. 1985 Sep;4(9):2301–2307. doi: 10.1002/j.1460-2075.1985.tb03930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y., Tabuchi A., Itoh Y., Katagiri H., Terawaki Y. Complete nucleotide sequence of mini-Rts1 and its copy mutant. J Bacteriol. 1984 Apr;158(1):307–312. doi: 10.1128/jb.158.1.307-312.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y., Wada C., Yura T. Roles of Escherichia coli heat shock proteins DnaK, DnaJ and GrpE in mini-F plasmid replication. Mol Gen Genet. 1990 Jan;220(2):277–282. doi: 10.1007/BF00260494. [DOI] [PubMed] [Google Scholar]
- Kusukawa N., Yura T. Heat shock protein GroE of Escherichia coli: key protective roles against thermal stress. Genes Dev. 1988 Jul;2(7):874–882. doi: 10.1101/gad.2.7.874. [DOI] [PubMed] [Google Scholar]
- Maki S., Miki T., Horiuchi T. DNA sequence of an amber replication mutant indicates that a 29 kd protein is the product of the F plasmid replication gene. Mol Gen Genet. 1984;194(1-2):337–339. doi: 10.1007/BF00383537. [DOI] [PubMed] [Google Scholar]
- Manis J. J., Kline B. C. F plasmid incompatibility and copy number genes: their map locations and interactions. Plasmid. 1978 Sep;1(4):492–507. doi: 10.1016/0147-619x(78)90007-0. [DOI] [PubMed] [Google Scholar]
- Masson L., Ray D. S. Mechanism of autonomous control of the Escherichia coli F plasmid: different complexes of the initiator/repressor protein are bound to its operator and to an F plasmid replication origin. Nucleic Acids Res. 1986 Jul 25;14(14):5693–5711. doi: 10.1093/nar/14.14.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murotsu T., Matsubara K., Sugisaki H., Takanami M. Nine unique repeating sequences in a region essential for replication and incompatibility of the mini-F plasmid. Gene. 1981 Nov;15(2-3):257–271. doi: 10.1016/0378-1119(81)90135-9. [DOI] [PubMed] [Google Scholar]
- Murotsu T., Tsutsui H., Matsubara K. Identification of the minimal essential region for the replication origin of miniF plasmid. Mol Gen Genet. 1984;196(2):373–378. doi: 10.1007/BF00328075. [DOI] [PubMed] [Google Scholar]
- Rokeach L. A., Søgaard-Andersen L., Molin S. Two functions of the E protein are key elements in the plasmid F replication control system. J Bacteriol. 1985 Dec;164(3):1262–1270. doi: 10.1128/jb.164.3.1262-1270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke R. W., Kline B. C., Trawick J. D., Ritts G. D. Genetic studies of F plasmid maintenance genes involved in copy number control, incompatability, and partitioning. Plasmid. 1982 Mar;7(2):163–179. doi: 10.1016/0147-619x(82)90075-0. [DOI] [PubMed] [Google Scholar]
- Shiba K., Ito K., Yura T. Suppressors of the secY24 mutation: identification and characterization of additional ssy genes in Escherichia coli. J Bacteriol. 1986 Jun;166(3):849–856. doi: 10.1128/jb.166.3.849-856.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard-Andersen L., Rokeach L. A., Molin S. Regulated expression of a gene important for replication of plasmid F in E. coli. EMBO J. 1984 Feb;3(2):257–262. doi: 10.1002/j.1460-2075.1984.tb01794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokino T., Murotsu T., Matsubara K. Purification and properties of the mini-F plasmid-encoded E protein needed for autonomous replication control of the plasmid. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4109–4113. doi: 10.1073/pnas.83.12.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolun A., Helinski D. R. Separation of the minimal replication region of the F plasmid into a replication origin segment and a trans-acting segment. Mol Gen Genet. 1982;186(3):372–377. doi: 10.1007/BF00729456. [DOI] [PubMed] [Google Scholar]
- Tsutsui H., Fujiyama A., Murotsu T., Matsubara K. Role of nine repeating sequences of the mini-F genome for expression of F-specific incompatibility phenotype and copy number control. J Bacteriol. 1983 Jul;155(1):337–344. doi: 10.1128/jb.155.1.337-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wada C., Akiyama Y., Ito K., Yura T. Inhibition of F plasmid replication in htpR mutants of Escherichia coli deficient in sigma 32 protein. Mol Gen Genet. 1986 May;203(2):208–213. doi: 10.1007/BF00333956. [DOI] [PubMed] [Google Scholar]
- Wada C., Imai M., Yura T. Host control of plasmid replication: requirement for the sigma factor sigma 32 in transcription of mini-F replication initiator gene. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8849–8853. doi: 10.1073/pnas.84.24.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson L. A., Phua S. H., Bergquist P. L., Lane H. E. An Mr 29000 protein is essential for mini-F maintenance in E. coli. Gene. 1982 Sep;19(2):173–178. doi: 10.1016/0378-1119(82)90003-8. [DOI] [PubMed] [Google Scholar]
- Zhou Y. N., Kusukawa N., Erickson J. W., Gross C. A., Yura T. Isolation and characterization of Escherichia coli mutants that lack the heat shock sigma factor sigma 32. J Bacteriol. 1988 Aug;170(8):3640–3649. doi: 10.1128/jb.170.8.3640-3649.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



