Abstract
An Escherichia coli F19 recA, nitrate reductase-deficient mutant was constructed by transposon mutagenesis and shown to be resistant to metronidazole. This mutant was a most suitable host for the isolation of Clostridium acetobutylicum genes on recombinant plasmids, which activated metronidazole and rendered the E. coli F19 strain sensitive to metronidazole. Twenty-five E. coli F19 clones containing different recombinant plasmids were isolated and classified into five groups on the basis of their sensitivity to metronidazole. The clones were tested for nitrate reductase, pyruvate-ferredoxin oxidoreductase, and hydrogenase activities. DNA hybridization and restriction endonuclease mapping revealed that four of the C. acetobutylicum insert DNA fragments on recombinant plasmids were linked in an 11.1-kb chromosomal fragment. DNA sequencing and amino acid homology studies indicated that this DNA fragment contained a flavodoxin gene which encoded a protein of 160 amino acids that activated metronidazole and made the E. coli F19 mutant very sensitive to metronidazole. The flavodoxin and hydrogenase genes which are involved in electron transfer systems were linked on the 11.1-kb DNA fragment from C. acetobutylicum.
Full text
PDF

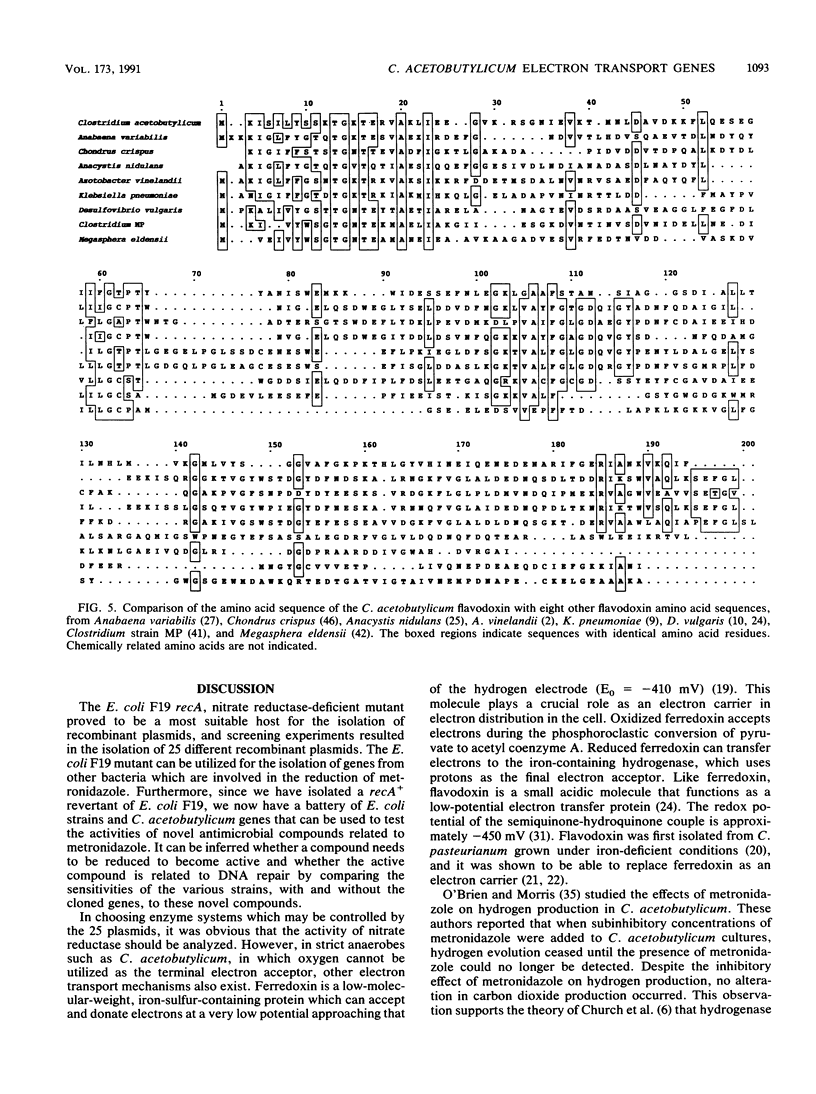


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allcock E. R., Reid S. J., Jones D. T., Woods D. R. Clostridium acetobutylicum Protoplast Formation and Regeneration. Appl Environ Microbiol. 1982 Mar;43(3):719–721. doi: 10.1128/aem.43.3.719-721.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett L. T., Jacobson M. R., Dean D. R. Isolation, sequencing, and mutagenesis of the nifF gene encoding flavodoxin from Azotobacter vinelandii. J Biol Chem. 1988 Jan 25;263(3):1364–1369. [PubMed] [Google Scholar]
- Chen J. S., Blanchard D. K. A simple hydrogenase-linked assay for ferredoxin and flavodoxin. Anal Biochem. 1979 Feb;93(1):216–222. [PubMed] [Google Scholar]
- Chrystal E. J., Koch R. L., McLafferty M. A., Goldman P. Relationship between metronidazole metabolism and bactericidal activity. Antimicrob Agents Chemother. 1980 Oct;18(4):566–573. doi: 10.1128/aac.18.4.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church D. L., Rabin H. R., Laishley E. J. Role of hydrogenase 1 of Clostridium pasteurianum in the reduction of metronidazole. Biochem Pharmacol. 1988 Apr 15;37(8):1525–1534. doi: 10.1016/0006-2952(88)90014-7. [DOI] [PubMed] [Google Scholar]
- Clark D., Cronan J. E., Jr Escherichia coli mutants with altered control of alcohol dehydrogenase and nitrate reductase. J Bacteriol. 1980 Jan;141(1):177–183. doi: 10.1128/jb.141.1.177-183.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond M. H. The base sequence of the nifF gene of Klebsiella pneumoniae and homology of the predicted amino acid sequence of its protein product to other flavodoxins. Biochem J. 1985 Dec 15;232(3):891–896. doi: 10.1042/bj2320891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubourdieu M., Fox J. L. Amino acid sequence of Desulfovibrio vulgaris flavodoxin. J Biol Chem. 1977 Feb 25;252(4):1453–1463. [PubMed] [Google Scholar]
- Edwards D. I., Mathison G. E. The mode of action of metronidazole against Trichomonas vaginalis. J Gen Microbiol. 1970 Nov;63(3):297–302. doi: 10.1099/00221287-63-3-297. [DOI] [PubMed] [Google Scholar]
- Gutierrez C., Barondess J., Manoil C., Beckwith J. The use of transposon TnphoA to detect genes for cell envelope proteins subject to a common regulatory stimulus. Analysis of osmotically regulated genes in Escherichia coli. J Mol Biol. 1987 May 20;195(2):289–297. doi: 10.1016/0022-2836(87)90650-4. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen P. J., Jones D. T., Woods D. R. Studies on Clostridium acetobutylicum glnA promoters and antisense RNA. Mol Microbiol. 1990 Sep;4(9):1575–1583. [PubMed] [Google Scholar]
- Jones D. T., Woods D. R. Acetone-butanol fermentation revisited. Microbiol Rev. 1986 Dec;50(4):484–524. doi: 10.1128/mr.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., van der Westhuizen A., Long S., Allcock E. R., Reid S. J., Woods D. R. Solvent Production and Morphological Changes in Clostridium acetobutylicum. Appl Environ Microbiol. 1982 Jun;43(6):1434–1439. doi: 10.1128/aem.43.6.1434-1439.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight E., Jr, D'Eustachio A. J., Hardy R. W. Flavodoxin: a flavoprotein with ferredoxin activity from Clostrium pasteurianum. Biochim Biophys Acta. 1966 Mar 7;113(3):626–628. doi: 10.1016/s0926-6593(66)80025-5. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr, Hardy R. W. Flavodoxin. Chemical and biological properties. J Biol Chem. 1967 Apr 10;242(7):1370–1374. [PubMed] [Google Scholar]
- Knight E., Jr, Hardy R. W. Isolation and characteristics of flavodoxin from nitrogen-fixing Clostridium pasteurianum. J Biol Chem. 1966 Jun 25;241(12):2752–2756. [PubMed] [Google Scholar]
- Knight R. C., Skolimowski I. M., Edwards D. I. The interaction of reduced metronidazole with DNA. Biochem Pharmacol. 1978;27(17):2089–2093. doi: 10.1016/0006-2952(78)90277-0. [DOI] [PubMed] [Google Scholar]
- Krey G. D., Vanin E. F., Swenson R. P. Cloning, nucleotide sequence, and expression of the flavodoxin gene from Desulfovibrio vulgaris (Hildenborough). J Biol Chem. 1988 Oct 25;263(30):15436–15443. [PubMed] [Google Scholar]
- Laudenbach D. E., Reith M. E., Straus N. A. Isolation, sequence analysis, and transcriptional studies of the flavodoxin gene from Anacystis nidulans R2. J Bacteriol. 1988 Jan;170(1):258–265. doi: 10.1128/jb.170.1.258-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., Patel P., Sankar P., Shanmugam K. T. Isolation and characterization of mutant strains of Escherichia coli altered in H2 metabolism. J Bacteriol. 1985 Apr;162(1):344–352. doi: 10.1128/jb.162.1.344-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt K. G., Straus N. A. Sequence of the flavodoxin gene from Anabaena variabilis 7120. Nucleic Acids Res. 1989 Jun 12;17(11):4384–4384. doi: 10.1093/nar/17.11.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narikawa S. Distribution of metronidazole susceptibility factors in obligate anaerobes. J Antimicrob Chemother. 1986 Nov;18(5):565–574. doi: 10.1093/jac/18.5.565. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- O'Brien R. W., Morris J. G. Effect of metronidazole on hydrogen production by Clostridium acetobutylicum. Arch Mikrobiol. 1972;84(3):225–233. doi: 10.1007/BF00425200. [DOI] [PubMed] [Google Scholar]
- O'Brien R. W., Morris J. G. Oxygen and the growth and metabolism of Clostridium acetobutylicum. J Gen Microbiol. 1971 Nov;68(3):307–318. doi: 10.1099/00221287-68-3-307. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholle R. R., Robb S. M., Robb F. T., Woods D. R. Nucleotide sequence and analysis of the Vibrio alginolyticus sucrase gene (scrB). Gene. 1989 Aug 1;80(1):49–56. doi: 10.1016/0378-1119(89)90249-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Yasunobu K. T., Mayhew S., Massey V. The primary structure of Peptostreptococcus elsdenii flavodoxin. J Biol Chem. 1973 Jun 25;248(12):4354–4366. [PubMed] [Google Scholar]
- Tanka M., Haniu M., Yasunobu F., Mayhew S. G. The amino acid sequence of the Clostridium MP flavodoxin. J Biol Chem. 1974 Jul 25;249(14):4393–4396. [PubMed] [Google Scholar]
- Voordouw G., Walker J. E., Brenner S. Cloning of the gene encoding the hydrogenase from Desulfovibrio vulgaris (Hildenborough) and determination of the NH2-terminal sequence. Eur J Biochem. 1985 May 2;148(3):509–514. doi: 10.1111/j.1432-1033.1985.tb08868.x. [DOI] [PubMed] [Google Scholar]
- Wahl R. C., Orme-Johnson W. H. Clostridial pyruvate oxidoreductase and the pyruvate-oxidizing enzyme specific to nitrogen fixation in Klebsiella pneumoniae are similar enzymes. J Biol Chem. 1987 Aug 5;262(22):10489–10496. [PubMed] [Google Scholar]
- Wakabayashi S., Kimura T., Fukuyama K., Matsubara H., Rogers L. J. The amino acid sequence of a flavodoxin from the eukaryotic red alga Chondrus crispus. Biochem J. 1989 Nov 1;263(3):981–984. doi: 10.1042/bj2630981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T. C., Beaulieu B. B., Jr, McLafferty M. A., Goldman P. Interaction of metronidazole with DNA repair mutants of Escherichia coli. Antimicrob Agents Chemother. 1984 Jan;25(1):65–70. doi: 10.1128/aac.25.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabeau M., Stanley K. K. Enhanced expression of cro-beta-galactosidase fusion proteins under the control of the PR promoter of bacteriophage lambda. EMBO J. 1982;1(10):1217–1224. doi: 10.1002/j.1460-2075.1982.tb00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappe H., Jones D. T., Woods D. R. Cloning and expression of Clostridium acetobutylicum endoglucanase, cellobiase and amino acid biosynthesis genes in Escherichia coli. J Gen Microbiol. 1986 May;132(5):1367–1372. doi: 10.1099/00221287-132-5-1367. [DOI] [PubMed] [Google Scholar]