Abstract
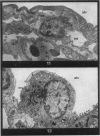
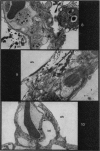
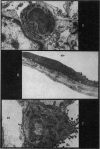
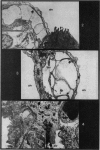
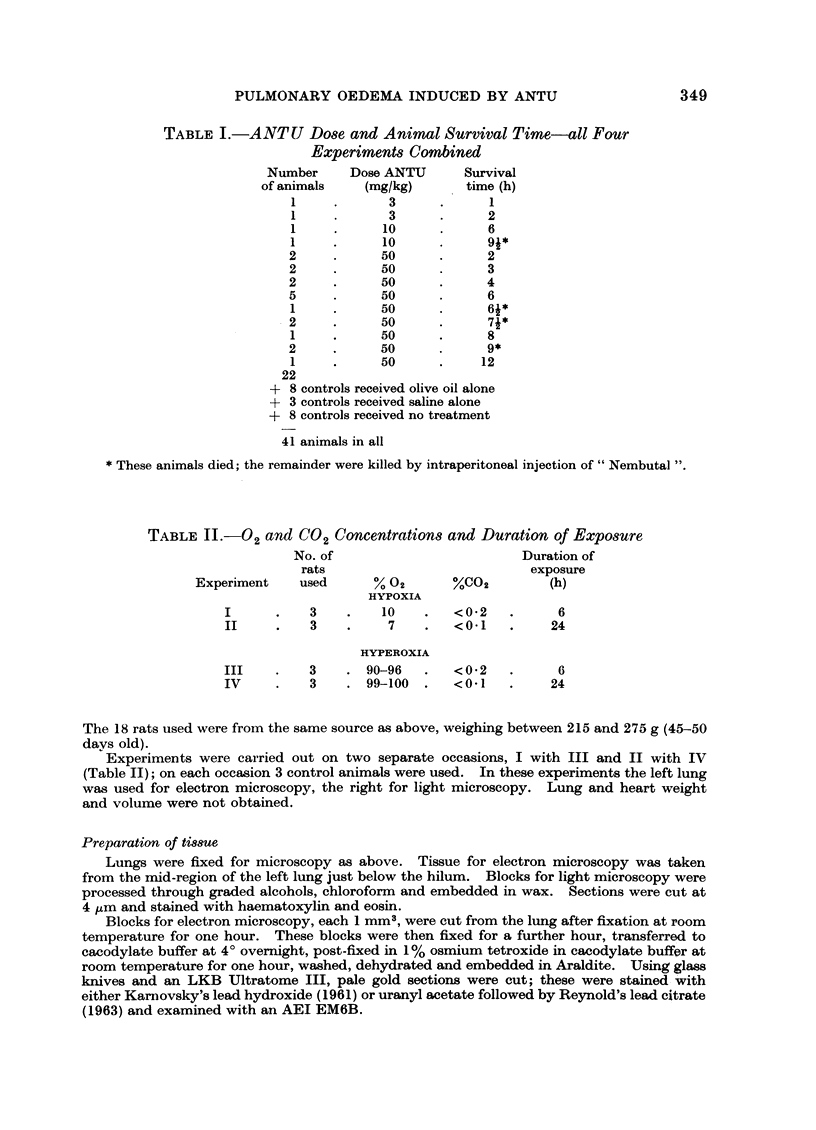
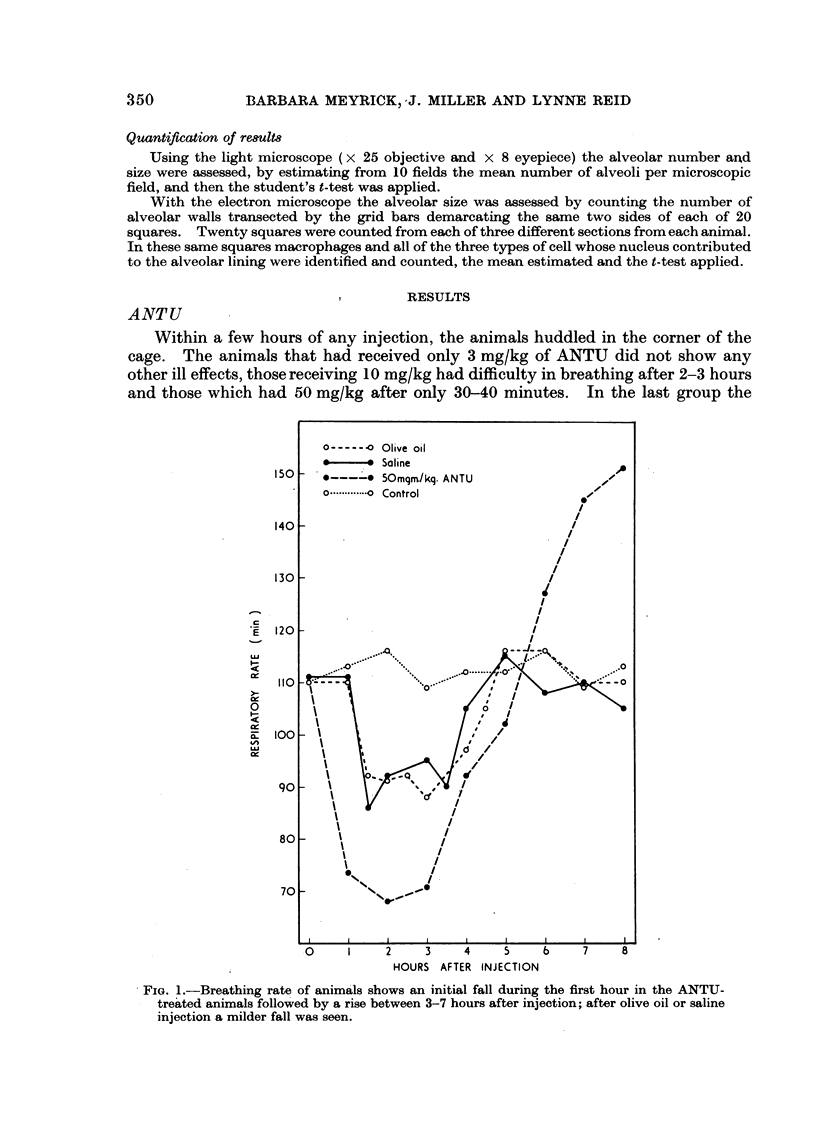
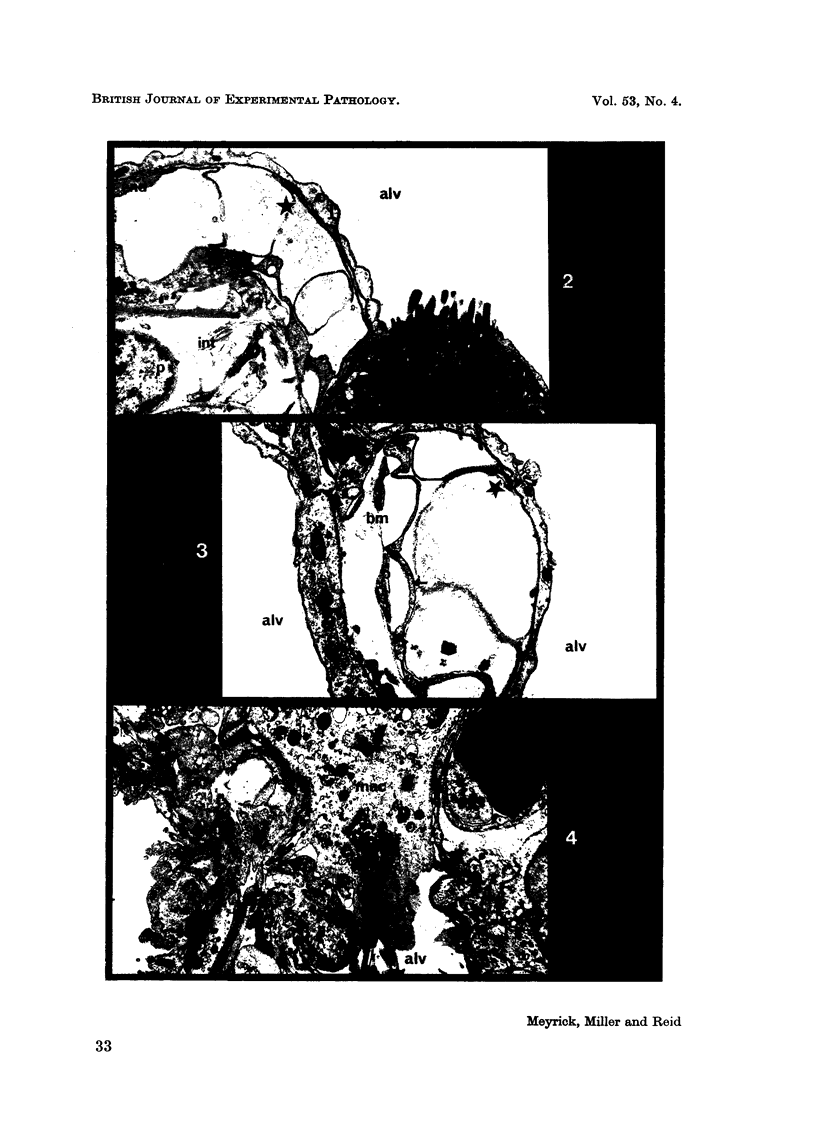
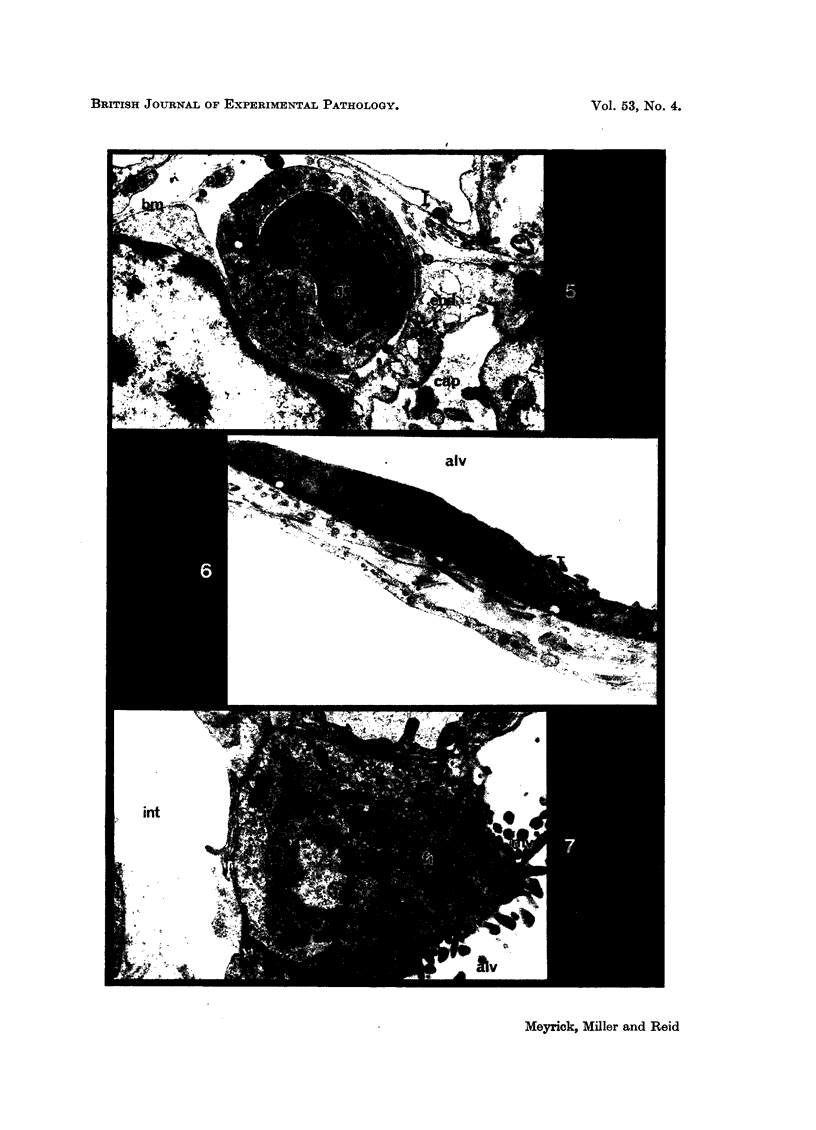
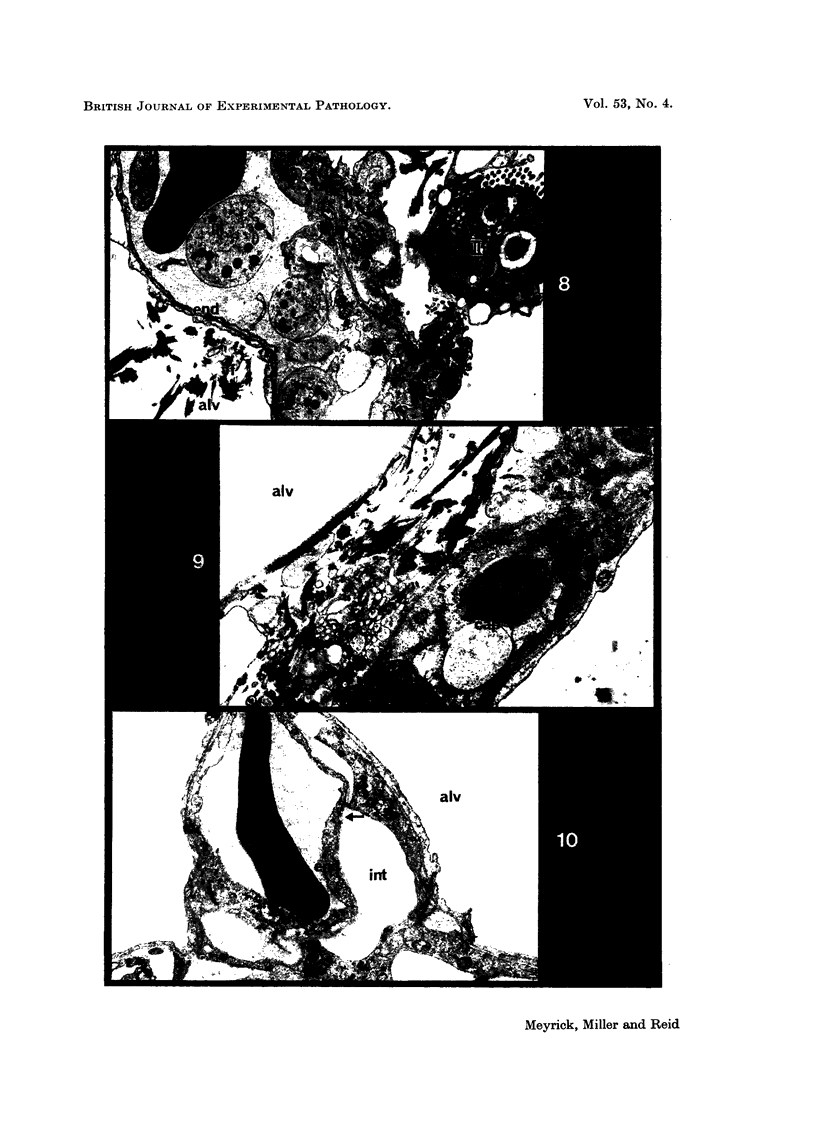
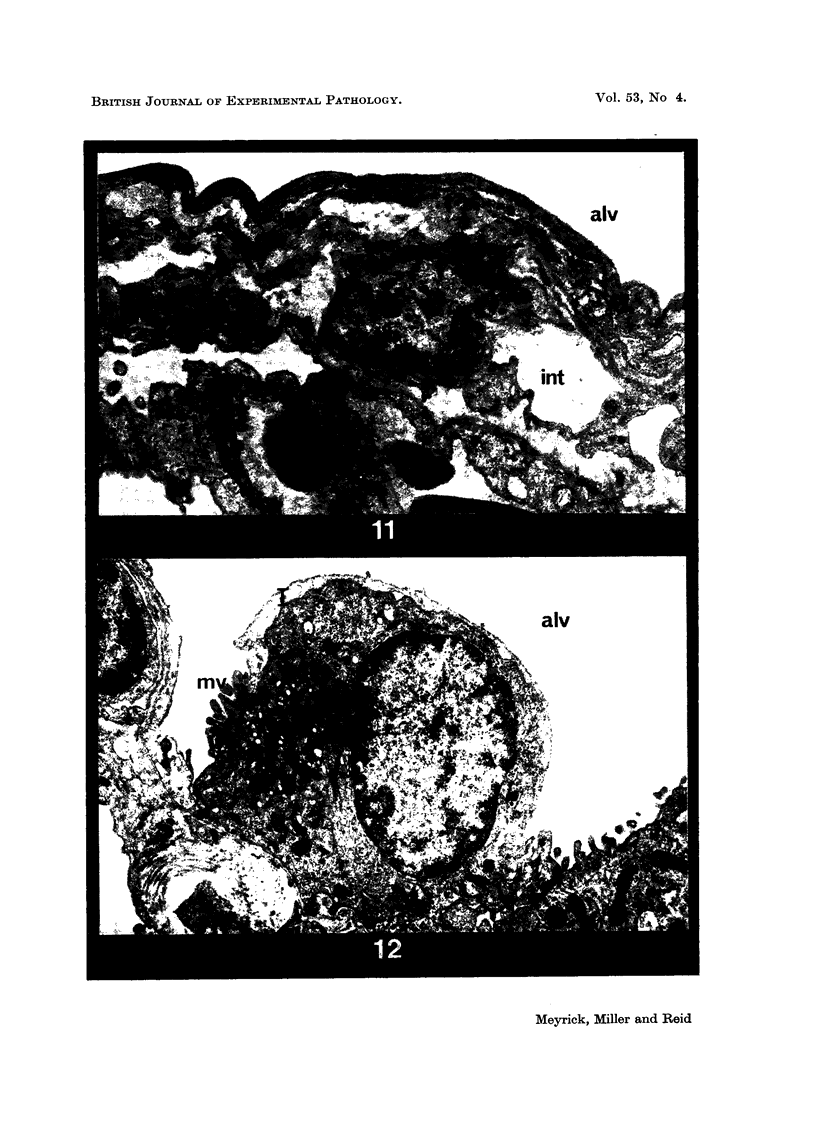
Pulmonary oedema has been experimentally induced in rats using either an intraperitoneal injection of alpha-naphthyl thiourea (ANTU) or by variation of atmospheric oxygen content. For the first time an electron microscopic study of the development of ANTU-induced lung oedema has been made. At 2 hours blebbing and scalloping of endothelial cells and interstitial oedema were observed, each showing increasing severity with increase of dose—3, 10 and 50 mg/kg doses. By 6 hours, in the 50 mg/kg treated animals, epithelial damage was also apparent. No alteration in mean alveolar size or in the distribution of alveolar lining cells was found. The breathing rate of the rats was slowed for the first 3 hours and then increased well above normal levels.
Hyperoxic conditions (99-100% O2 at 1 atm/pressure) produced interstitial oedema by 6 hours, followed at 24 hours by both endothelial and epithelial damage. Hypoxic conditions (7-10% O2 at 1 atm/pressure) did not produce these effects. Both hyperoxic and hypoxic conditions were associated with a significant increase in the mean alveolar size, an apparent decrease in number of Type III pneumonocytes and in the macrophages when related to alveolar cell number, while the counts of Types I and II pneumonocytes remained within the normal range.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H., Wyatt J. P. Oxygen poisoning in mice. Ultrastructural and surfactant studies during exposure and recovery. Arch Pathol. 1970 Nov;90(5):463–472. [PubMed] [Google Scholar]
- Alexander I. G. The ultrastructure of the pulmonary alveolar vessels in Mendelson's (acid pulmonary aspiration) syndrome. Br J Anaesth. 1968 Jun;40(6):408–414. doi: 10.1093/bja/40.6.408. [DOI] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y., Wyatt J. P. Reaction of the lung cells to a high concentration of oxygen. Arch Pathol. 1968 Dec;86(6):671–675. [PubMed] [Google Scholar]
- Burri P. H., Weibel E. R. Morphometric estimation of pulmonary diffusion capacity. II. Effect of Po2 on the growing lung, adaption of the growing rat lung to hypoxia and hyperoxia. Respir Physiol. 1971 Jan;11(2):247–264. doi: 10.1016/0034-5687(71)90028-4. [DOI] [PubMed] [Google Scholar]
- Böhm G. M. Vascular permeability changes during experimentally produced pulmonary oedema in rats. J Pathol Bacteriol. 1966 Jul;92(1):151–161. doi: 10.1002/path.1700920116. [DOI] [PubMed] [Google Scholar]
- COOK C. D., MEAD J., SCHREINER G. L., FRANK N. R., CRAIG J. M. Pulmonary mechanics during induced pulmonary edema in anesthetized dogs. J Appl Physiol. 1959 Mar;14(2):177–186. doi: 10.1152/jappl.1959.14.2.177. [DOI] [PubMed] [Google Scholar]
- Coalson J. J., Beller J. J., Greenfield L. J. Effects of 100 per cent. oxygen ventilation on pulmonary ultrastructure and mechanics. J Pathol. 1971 Aug;104(4):267–273. doi: 10.1002/path.1711040409. [DOI] [PubMed] [Google Scholar]
- Cottrell T. S., Levine O. R., Senior R. M., Wiener J., Spiro D., Fishman A. P. Electron microscopic alterations at the alveolar level in pulmonary edema. Circ Res. 1967 Dec;21(6):783–797. doi: 10.1161/01.res.21.6.783. [DOI] [PubMed] [Google Scholar]
- HESSE F. E., LOOSLI C. G. The lining of the alveoli in mice, rats, dogs, and frogs following acute pulmonary edema produced by ANTU poisoning. Anat Rec. 1949 Oct;105(2):299-323, incl 6 pl. doi: 10.1002/ar.1091050207. [DOI] [PubMed] [Google Scholar]
- Hayes J. A., Shiga A. Ultrastructural changes in pulmonary oedema produced experimentally with ammonium sulphate. J Pathol. 1970 Apr;100(4):281–286. doi: 10.1002/path.1711000407. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J. Simple methods for "staining with lead" at high pH in electron microscopy. J Biophys Biochem Cytol. 1961 Dec;11:729–732. doi: 10.1083/jcb.11.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapanci Y., Weibel E. R., Kaplan H. P., Robinson F. R. Pathogenesis and reversibility of the pulmonary lesions of oxygen toxicity in monkeys. II. Ultrastructural and morphometric studies. Lab Invest. 1969 Jan;20(1):101–118. [PubMed] [Google Scholar]
- Kisch B. Electron microscopy of capillary hemorrhage. I. Post hypoxemia hemorrhage in the lungs. Exp Med Surg. 1965;23(1):117–126. [PubMed] [Google Scholar]
- Kistler G. S., Caldwell P. R., Weibel E. R. Development of fine structural damage to alveolar and capillary lining cells in oxygen-poisoned rat lungs. J Cell Biol. 1967 Mar;32(3):605–628. doi: 10.1083/jcb.32.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOW F. N. The pulmonary alveolar epithelium of laboratory mammals and man. Anat Rec. 1953 Oct;117(2):241–263. doi: 10.1002/ar.1091170208. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Reid L. The alveolar brush cell in rat lung--a third pneumonocyte. J Ultrastruct Res. 1968 Apr;23(1):71–80. doi: 10.1016/s0022-5320(68)80032-2. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Reid L. The alveolar wall. Br J Dis Chest. 1970 Jul;64(3):121–140. doi: 10.1016/s0007-0971(70)80001-8. [DOI] [PubMed] [Google Scholar]
- Milic-Emili J., Ruff F. Effects of pulmonary congestion and edema on the small airways. Bull Physiopathol Respir (Nancy) 1971 Nov-Dec;7(6):1181–1196. [PubMed] [Google Scholar]
- RECH R. H., BORISON H. L. Vagotomy-induced pulmonary edema in the guinea pig. Am J Physiol. 1962 Mar;202:499–504. doi: 10.1152/ajplegacy.1962.202.3.499. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHTER C. P. The physiology and cytology of pulmonary edema and pleural effusion produced in rats by alpha-naphthyl thiourea (ANTU). J Thorac Surg. 1952 Jan;23(1):66–91. [PubMed] [Google Scholar]
- Reidbord H. E., Spitz W. U. Ultrastructural alterations in rat lungs. Changes following asphyxiation. Arch Pathol. 1966 Jul;82(1):80–84. [PubMed] [Google Scholar]
- SCHAEFER K. E., AVERY M. E., BENSCH K. TIME COURSE OF CHANGES IN SURFACE TENSION AND MORPHOLOGY OF ALVEOLAR EPITHELIAL CELLS IN CO2-INDUCED HYALINE MEMBRANE DISEASE. J Clin Invest. 1964 Nov;43:2080–2093. doi: 10.1172/JCI105082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub N. C., Nagano H., Pearce M. L. Pulmonary edema in dogs, especially the sequence of fluid accumulation in lungs. J Appl Physiol. 1967 Feb;22(2):227–240. doi: 10.1152/jappl.1967.22.2.227. [DOI] [PubMed] [Google Scholar]
- Teplitz C. The ultrastructural basis for pulmonary pathophysiology following trauma. Pathogenesis of pulmonary edema. J Trauma. 1968 Sep;8(5):700–714. doi: 10.1097/00005373-196809000-00009. [DOI] [PubMed] [Google Scholar]