Abstract
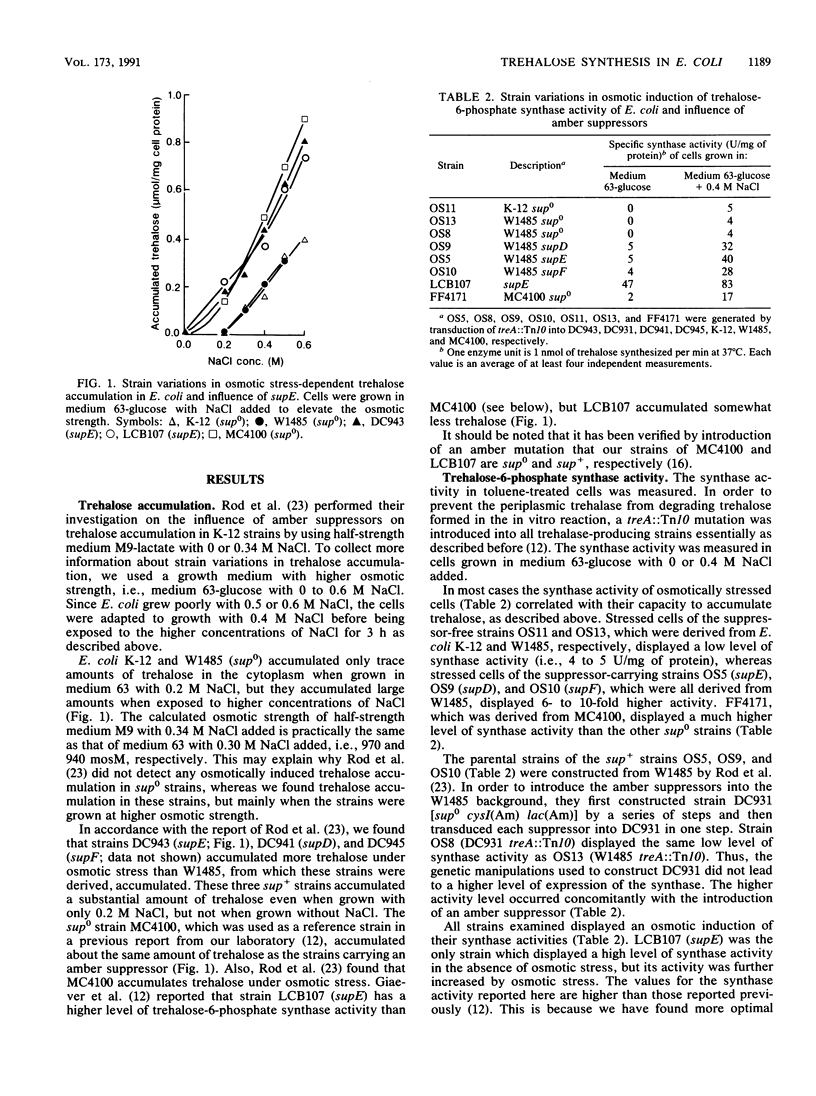
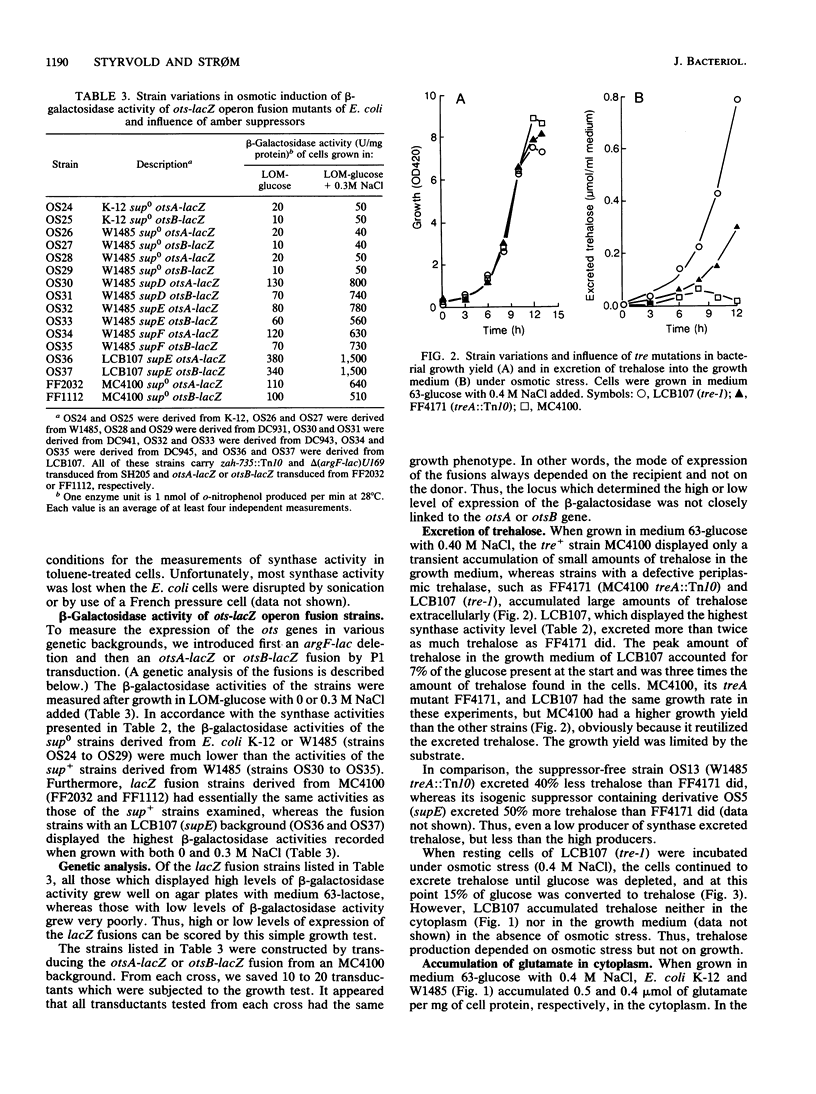
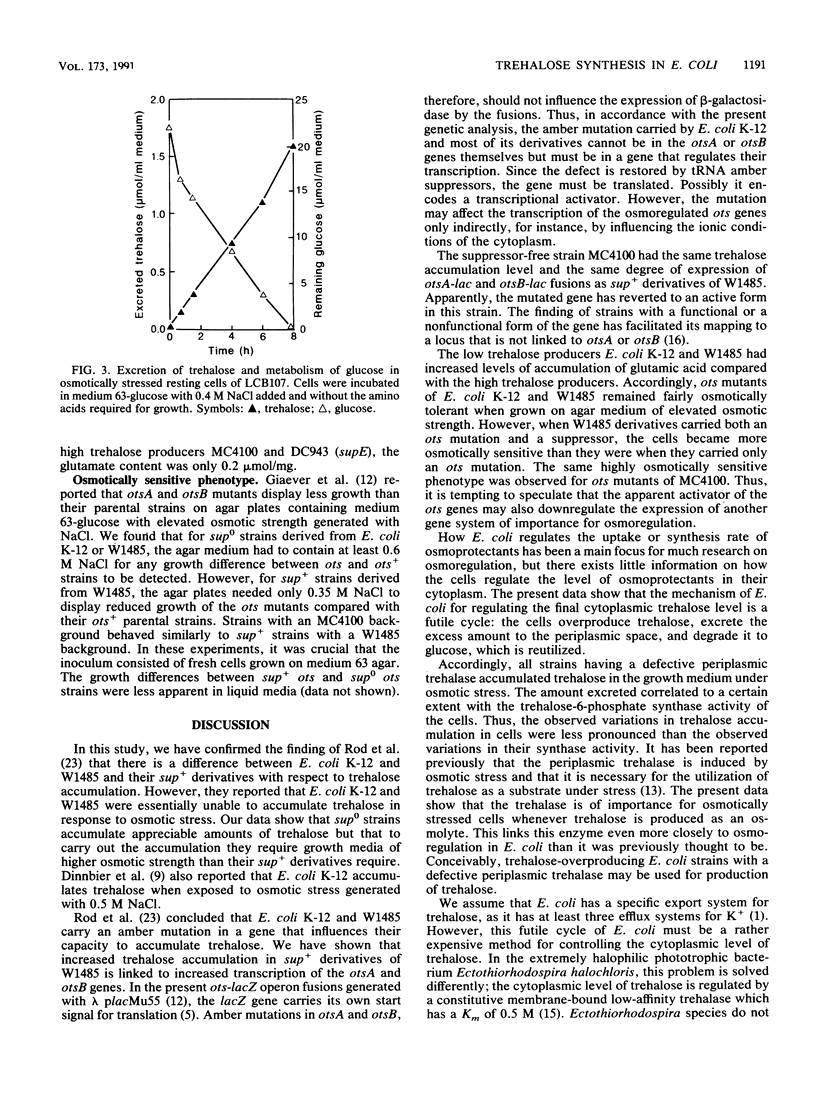
It has been reported previously that Escherichia coli K-12 carries an amber mutation that prevents osmotic stress-dependent accumulation of trehalose (M. L. Rod, K. Y. Alam, P. R. Cunningham, and D. P. Clark, J. Bacteriol. 170:3601-3610, 1988). We report that E. coli K-12 and W1485 (sup0) accumulated trehalose but that they required a higher osmotic strength in the growth medium than that required by their sup+ derivatives. Furthermore, the sup+ derivatives displayed both strongly increased trehalose-6-phosphate synthase activity and expression of otsA-lacZ and otsB-lacZ operon fusions compared with their parental strains. It is suggested that the amber mutation in question may be in a gene system encoding a transcriptional activator of the ots genes which govern the synthase. The much-used sup0 strain MC4100 behaved like the sup+ derivatives of W1485 with respect to trehalose synthesis. treA mutants with a defective periplasmic trehalase accumulated trehalose extracellularly under osmotic stress. The amount of trehalose excreted correlated with their synthase activity. Strains with an intact trehalase did not display extracellular trehalose accumulation. Thus, stressed E. coli cells regulate the cytoplasmic level of trehalose by a futile cycle involving overproduction, excretion, and degradation to glucose, which is reutilized.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker E. P., Booth I. R., Dinnbier U., Epstein W., Gajewska A. Evidence for multiple K+ export systems in Escherichia coli. J Bacteriol. 1987 Aug;169(8):3743–3749. doi: 10.1128/jb.169.8.3743-3749.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W., Ehmann U., Bremer E., Middendorf A., Postma P. Trehalase of Escherichia coli. Mapping and cloning of its structural gene and identification of the enzyme as a periplasmic protein induced under high osmolarity growth conditions. J Biol Chem. 1987 Sep 25;262(27):13212–13218. [PubMed] [Google Scholar]
- Boos W., Ehmann U., Forkl H., Klein W., Rimmele M., Postma P. Trehalose transport and metabolism in Escherichia coli. J Bacteriol. 1990 Jun;172(6):3450–3461. doi: 10.1128/jb.172.6.3450-3461.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer E., Silhavy T. J., Weinstock G. M. Transposable lambda placMu bacteriophages for creating lacZ operon fusions and kanamycin resistance insertions in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1092–1099. doi: 10.1128/jb.162.3.1092-1099.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairney J., Booth I. R., Higgins C. F. Osmoregulation of gene expression in Salmonella typhimurium: proU encodes an osmotically induced betaine transport system. J Bacteriol. 1985 Dec;164(3):1224–1232. doi: 10.1128/jb.164.3.1224-1232.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. A third L-proline permease in Salmonella typhimurium which functions in media of elevated osmotic strength. J Bacteriol. 1982 Sep;151(3):1433–1443. doi: 10.1128/jb.151.3.1433-1443.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnbier U., Limpinsel E., Schmid R., Bakker E. P. Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K-12 to elevated sodium chloride concentrations. Arch Microbiol. 1988;150(4):348–357. doi: 10.1007/BF00408306. [DOI] [PubMed] [Google Scholar]
- Eshoo M. W. lac fusion analysis of the bet genes of Escherichia coli: regulation by osmolarity, temperature, oxygen, choline, and glycine betaine. J Bacteriol. 1988 Nov;170(11):5208–5215. doi: 10.1128/jb.170.11.5208-5215.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever H. M., Styrvold O. B., Kaasen I., Strøm A. R. Biochemical and genetic characterization of osmoregulatory trehalose synthesis in Escherichia coli. J Bacteriol. 1988 Jun;170(6):2841–2849. doi: 10.1128/jb.170.6.2841-2849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez C., Ardourel M., Bremer E., Middendorf A., Boos W., Ehmann U. Analysis and DNA sequence of the osmoregulated treA gene encoding the periplasmic trehalase of Escherichia coli K12. Mol Gen Genet. 1989 Jun;217(2-3):347–354. doi: 10.1007/BF02464903. [DOI] [PubMed] [Google Scholar]
- Landfald B., Strøm A. R. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J Bacteriol. 1986 Mar;165(3):849–855. doi: 10.1128/jb.165.3.849-855.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. I., Sydnes L. K., Landfald B., Strøm A. R. Osmoregulation in Escherichia coli by accumulation of organic osmolytes: betaines, glutamic acid, and trehalose. Arch Microbiol. 1987 Feb;147(1):1–7. doi: 10.1007/BF00492896. [DOI] [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Maréchal L. R. Transport and metabolism of trehalose in Escherichia coli and Salmonella typhimurium. Arch Microbiol. 1984 Jan;137(1):70–73. doi: 10.1007/BF00425810. [DOI] [PubMed] [Google Scholar]
- Perroud B., Le Rudulier D. Glycine betaine transport in Escherichia coli: osmotic modulation. J Bacteriol. 1985 Jan;161(1):393–401. doi: 10.1128/jb.161.1.393-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rod M. L., Alam K. Y., Cunningham P. R., Clark D. P. Accumulation of trehalose by Escherichia coli K-12 at high osmotic pressure depends on the presence of amber suppressors. J Bacteriol. 1988 Aug;170(8):3601–3610. doi: 10.1128/jb.170.8.3601-3610.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer H., Boos W. Transfer of the delta (argF-lac)U169 mutation between Escherichia coli strains by selection for a closely linked Tn10 insertion. Mol Gen Genet. 1983;192(1-2):293–294. doi: 10.1007/BF00327683. [DOI] [PubMed] [Google Scholar]
- de Lares L. B., Ratouchniak J., Casse F. Chromosomal location of gene governing the trehalose utilization in Escherichia coli K12. Mol Gen Genet. 1977 Mar 28;152(1):105–108. doi: 10.1007/BF00264946. [DOI] [PubMed] [Google Scholar]


