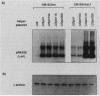
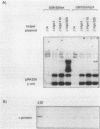
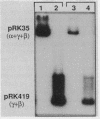
Abstract
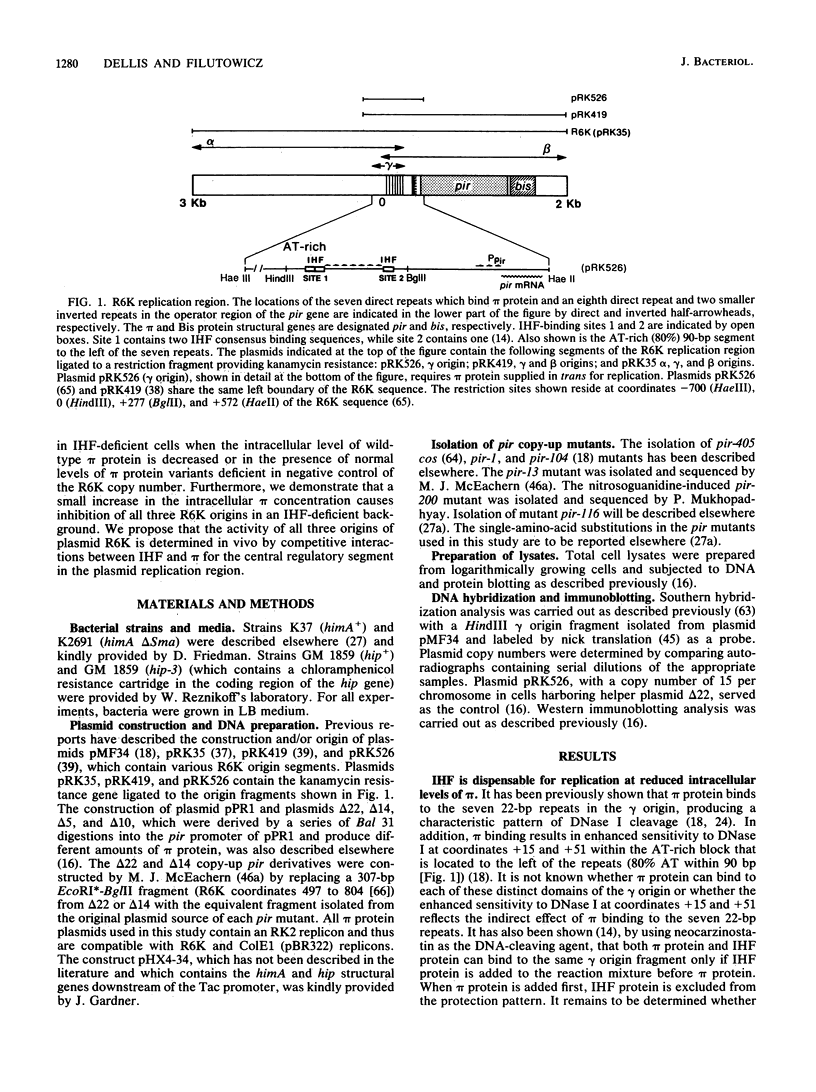
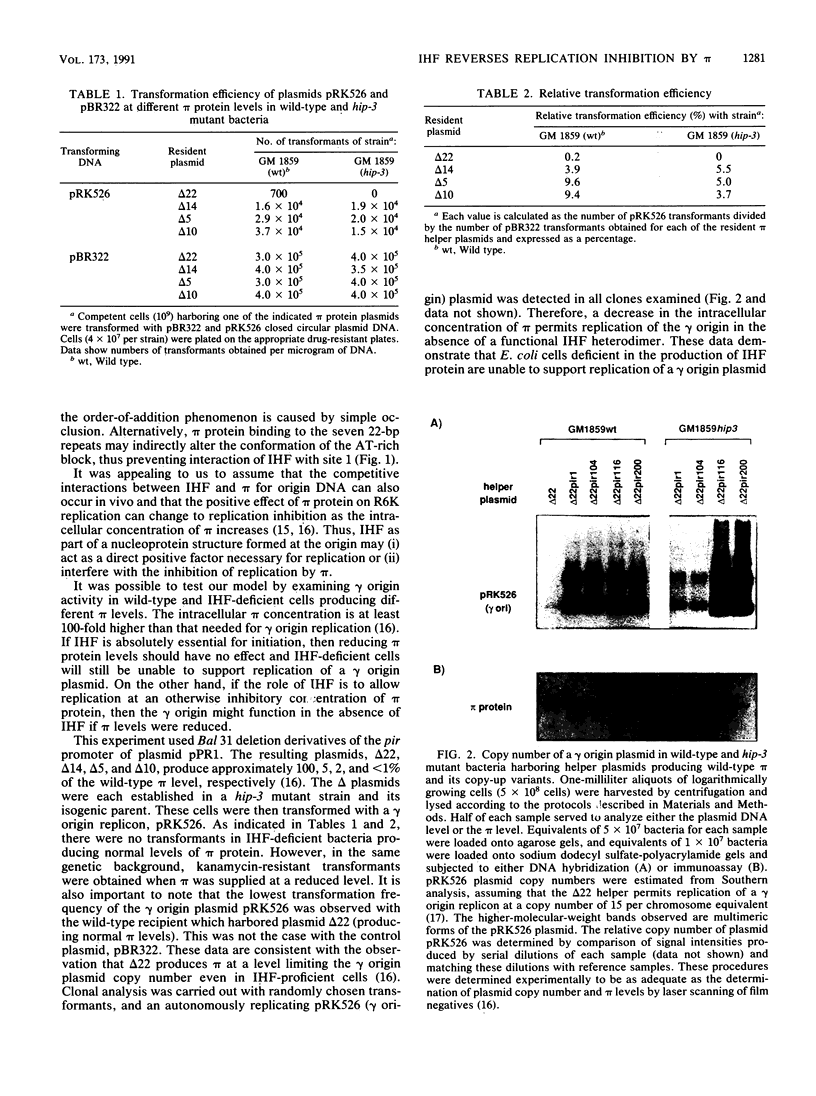
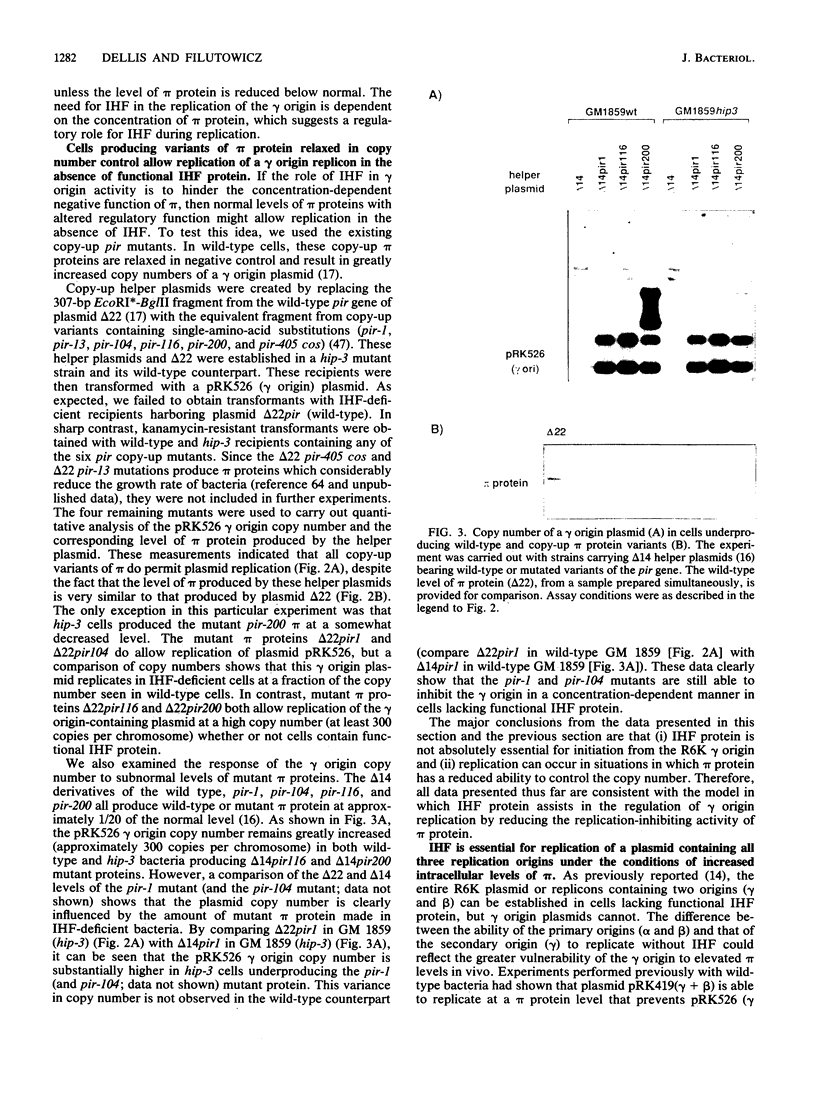
Integration host factor (IHF) protein is the only host-encoded protein known to bind and to affect replication of the gamma origin of Escherichia coli plasmid R6K. We examined the ability of R6K origins to replicate in cells lacking either of the two subunits of IHF. As shown previously, the gamma origin cannot replicate in IHF-deficient cells. However, this inability to replicate was relieved under the following conditions: underproduction of the wild-type pi replication protein of R6K or production of normal levels of mutant pi proteins which exhibit relaxed replication control. The copy number of plasmids containing the primary R6K origins (alpha and beta) is substantially reduced in IHF-deficient bacteria. Furthermore, replication of these plasmids is completely inhibited if the IHF-deficient strains contain a helper plasmid producing additional wild-type pi protein. IHF protein has previously been shown to bind to two sites within the gamma origin. These sites flank a central repeat segment which binds pi protein. We propose a model in which IHF binding to its sites reduces the replication inhibitor activity of pi protein at all three R6K origins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bear S. E., Clemens J. B., Enquist L. W., Zagursky R. J. Mutational analysis of the lambda int gene: DNA sequence of dominant mutations. J Bacteriol. 1987 Dec;169(12):5880–5883. doi: 10.1128/jb.169.12.5880-5883.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear S. E., Court D. L., Friedman D. I. An accessory role for Escherichia coli integration host factor: characterization of a lambda mutant dependent upon integration host factor for DNA packaging. J Virol. 1984 Dec;52(3):966–972. doi: 10.1128/jvi.52.3.966-972.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biek D. P., Cohen S. N. Involvement of integration host factor (IHF) in maintenance of plasmid pSC101 in Escherichia coli: characterization of pSC101 mutants that replicate in the absence of IHF. J Bacteriol. 1989 Apr;171(4):2056–2065. doi: 10.1128/jb.171.4.2056-2065.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biek D. P., Cohen S. N. Involvement of integration host factor (IHF) in maintenance of plasmid pSC101 in Escherichia coli: mutations in the topA gene allow pSC101 replication in the absence of IHF. J Bacteriol. 1989 Apr;171(4):2066–2074. doi: 10.1128/jb.171.4.2066-2074.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. A model for initiation at origins of DNA replication. Cell. 1988 Sep 23;54(7):915–918. doi: 10.1016/0092-8674(88)90102-x. [DOI] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell. 1988 Mar 11;52(5):743–755. doi: 10.1016/0092-8674(88)90412-6. [DOI] [PubMed] [Google Scholar]
- Craig N. L., Nash H. A. E. coli integration host factor binds to specific sites in DNA. Cell. 1984 Dec;39(3 Pt 2):707–716. doi: 10.1016/0092-8674(84)90478-1. [DOI] [PubMed] [Google Scholar]
- Crosa J. H., Luttropp L. K., Heffron F., Falkow S. Two replication initiation sites on R-plasmid DNA. Mol Gen Genet. 1975 Sep 15;140(1):39–50. doi: 10.1007/BF00268987. [DOI] [PubMed] [Google Scholar]
- Crosa J. H. Three origins of replication are active in vivo in the R plasmid RSF1040. J Biol Chem. 1980 Dec 10;255(23):11075–11077. [PubMed] [Google Scholar]
- Dasgupta S., Masukata H., Tomizawa J. Multiple mechanisms for initiation of ColE1 DNA replication: DNA synthesis in the presence and absence of ribonuclease H. Cell. 1987 Dec 24;51(6):1113–1122. doi: 10.1016/0092-8674(87)90597-6. [DOI] [PubMed] [Google Scholar]
- Echols H. Multiple DNA-protein interactions governing high-precision DNA transactions. Science. 1986 Sep 5;233(4768):1050–1056. doi: 10.1126/science.2943018. [DOI] [PubMed] [Google Scholar]
- Feiss M., Fogarty S., Christiansen S. Bacteriophage lambda DNA packaging: a mutant terminase that is independent of integration host factor. Mol Gen Genet. 1988 Apr;212(1):142–148. doi: 10.1007/BF00322457. [DOI] [PubMed] [Google Scholar]
- Feiss M., Frackman S., Sippy J. Essential interaction between lambdoid phage 21 terminase and the Escherichia coli integrative host factor. J Mol Biol. 1985 May 25;183(2):239–246. doi: 10.1016/0022-2836(85)90216-5. [DOI] [PubMed] [Google Scholar]
- Filutowicz M., Appelt K. The integration host factor of Escherichia coli binds to multiple sites at plasmid R6K gamma origin and is essential for replication. Nucleic Acids Res. 1988 May 11;16(9):3829–3843. doi: 10.1093/nar/16.9.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filutowicz M., McEachern M. J., Helinski D. R. Positive and negative roles of an initiator protein at an origin of replication. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9645–9649. doi: 10.1073/pnas.83.24.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filutowicz M., McEachern M. J., Mukhopadhyay P., Greener A., Yang S. L., Helinski D. R. DNA and protein interactions in the regulation of plasmid replication. J Cell Sci Suppl. 1987;7:15–31. doi: 10.1242/jcs.1987.supplement_7.2. [DOI] [PubMed] [Google Scholar]
- Filutowicz M., McEachern M., Greener A., Mukhopadhyay P., Uhlenhopp E., Durland R., Helinski D. Role of the pi initiation protein and direct nucleotide sequence repeats in the regulation of plasmid R6K replication. Basic Life Sci. 1985;30:125–140. doi: 10.1007/978-1-4613-2447-8_13. [DOI] [PubMed] [Google Scholar]
- Filutowicz M., Roll J. The requirement of IHF protein for extrachromosomal replication of the Escherichia coli oriC in a mutant deficient in DNA polymerase I activity. New Biol. 1990 Sep;2(9):818–827. [PubMed] [Google Scholar]
- Filutowicz M., Uhlenhopp E., Helinski D. R. Binding of purified wild-type and mutant pi initiation proteins to a replication origin region of plasmid R6K. J Mol Biol. 1986 Jan 20;187(2):225–239. doi: 10.1016/0022-2836(86)90230-5. [DOI] [PubMed] [Google Scholar]
- Flamm E. L., Weisberg R. A. Primary structure of the hip gene of Escherichia coli and of its product, the beta subunit of integration host factor. J Mol Biol. 1985 May 25;183(2):117–128. doi: 10.1016/0022-2836(85)90206-2. [DOI] [PubMed] [Google Scholar]
- Friedman D. I. Integration host factor: a protein for all reasons. Cell. 1988 Nov 18;55(4):545–554. doi: 10.1016/0092-8674(88)90213-9. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. J., Carver D., Gellert M. Synergistic effect of himA and gyrB mutations: evidence that him functions control expression of ilv and xyl genes. J Bacteriol. 1984 Feb;157(2):484–489. doi: 10.1128/jb.157.2.484-489.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell B. E. Participation of Escherichia coli integration host factor in the P1 plasmid partition system. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6657–6661. doi: 10.1073/pnas.85.18.6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamas P., Burger A. C., Churchward G., Caro L., Galas D., Chandler M. Replication of pSC101: effects of mutations in the E. coli DNA binding protein IHF. Mol Gen Genet. 1986 Jul;204(1):85–89. doi: 10.1007/BF00330192. [DOI] [PubMed] [Google Scholar]
- Germino J., Bastia D. Interaction of the plasmid R6K-encoded replication initiator protein with its binding sites on DNA. Cell. 1983 Aug;34(1):125–134. doi: 10.1016/0092-8674(83)90142-3. [DOI] [PubMed] [Google Scholar]
- Germino J., Bastia D. Rapid purification of a cloned gene product by genetic fusion and site-specific proteolysis. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4692–4696. doi: 10.1073/pnas.81.15.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. D., Nash H. A. Functional replacement of a protein-induced bend in a DNA recombination site. Nature. 1989 Sep 21;341(6239):251–254. doi: 10.1038/341251a0. [DOI] [PubMed] [Google Scholar]
- Granston A. E., Alessi D. M., Eades L. J., Friedman D. I. A point mutation in the Nul gene of bacteriophage lambda facilitates phage growth in Escherichia coli with himA and gyrB mutations. Mol Gen Genet. 1988 Apr;212(1):149–156. doi: 10.1007/BF00322458. [DOI] [PubMed] [Google Scholar]
- Greenstein D., Zinder N. D., Horiuchi K. Integration host factor interacts with the DNA replication enhancer of filamentous phage f1. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6262–6266. doi: 10.1073/pnas.85.17.6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins N. P., Collier D. A., Kilpatrick M. W., Krause H. M. Supercoiling and integration host factor change the DNA conformation and alter the flow of convergent transcription in phage Mu. J Biol Chem. 1989 Feb 15;264(5):3035–3042. [PubMed] [Google Scholar]
- Inuzuka M., Helinski D. R. Replication of antibiotic resistance plasmid R6K DNA in vitro. Biochemistry. 1978 Jun 27;17(13):2567–2573. doi: 10.1021/bi00606a017. [DOI] [PubMed] [Google Scholar]
- Inuzuka M., Helinski D. R. Requirement of a plasmid-encoded protein for replication in vitro of plasmid R6K. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5381–5385. doi: 10.1073/pnas.75.11.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inuzuka N., Inuzuka M., Helinski D. R. Activity in vitro of three replication origins of the antibiotic resistance plasmid RSF1040. J Biol Chem. 1980 Dec 10;255(23):11071–11074. [PubMed] [Google Scholar]
- Kogoma T. A novel Escherichia coli mutant capable of DNA replication in the absence of protein synthesis. J Mol Biol. 1978 May 5;121(1):55–69. doi: 10.1016/0022-2836(78)90262-0. [DOI] [PubMed] [Google Scholar]
- Kogoma T. Absence of RNase H allows replication of pBR322 in Escherichia coli mutants lacking DNA polymerase I. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7845–7849. doi: 10.1073/pnas.81.24.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogoma T., von Meyenburg K. The origin of replication, oriC, and the dnaA protein are dispensable in stable DNA replication (sdrA) mutants of Escherichia coli K-12. EMBO J. 1983;2(3):463–468. doi: 10.1002/j.1460-2075.1983.tb01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter R., Helinski D. R. Activity of the replication terminus of plasmid R6K in hybrid replicons in Escherichia coli. J Mol Biol. 1978 Sep 25;124(3):425–441. doi: 10.1016/0022-2836(78)90180-8. [DOI] [PubMed] [Google Scholar]
- Kolter R., Helinski D. R. Construction of plasmid R6K derivatives in vitro: characterization of the R6K replication region. Plasmid. 1978 Sep;1(4):571–580. doi: 10.1016/0147-619x(78)90014-8. [DOI] [PubMed] [Google Scholar]
- Kolter R., Inuzuka M., Helinski D. R. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell. 1978 Dec;15(4):1199–1208. doi: 10.1016/0092-8674(78)90046-6. [DOI] [PubMed] [Google Scholar]
- Krause H. M., Higgins N. P. Positive and negative regulation of the Mu operator by Mu repressor and Escherichia coli integration host factor. J Biol Chem. 1986 Mar 15;261(8):3744–3752. [PubMed] [Google Scholar]
- Kur J., Hasan N., Szybalski W. Physical and biological consequences of interactions between integration host factor (IHF) and coliphage lambda late p'R promoter and its mutants. Gene. 1989 Sep 1;81(1):1–15. doi: 10.1016/0378-1119(89)90331-4. [DOI] [PubMed] [Google Scholar]
- Lange-Gustafson B. J., Nash H. A. Purification and properties of Int-h, a variant protein involved in site-specific recombination of bacteriophage lambda. J Biol Chem. 1984 Oct 25;259(20):12724–12732. [PubMed] [Google Scholar]
- Lovett M. A., Sparks R. B., Helinski D. R. Bidirectional replication of plasmid R6K DNA in Escherichia coli; correspondence between origin of replication and position of single-strand break in relaxed complex. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2905–2909. doi: 10.1073/pnas.72.8.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajna J., Oppenheim A. B., Rattray A., Gottesman M. Translation initiation of bacteriophage lambda gene cII requires integration host factor. J Bacteriol. 1986 Jan;165(1):167–174. doi: 10.1128/jb.165.1.167-174.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masukata H., Dasgupta S., Tomizawa J. Transcriptional activation of ColE1 DNA synthesis by displacement of the nontranscribed strand. Cell. 1987 Dec 24;51(6):1123–1130. doi: 10.1016/0092-8674(87)90598-8. [DOI] [PubMed] [Google Scholar]
- McEachern M. J., Bott M. A., Tooker P. A., Helinski D. R. Negative control of plasmid R6K replication: possible role of intermolecular coupling of replication origins. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7942–7946. doi: 10.1073/pnas.86.20.7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachern M. J., Filutowicz M., Helinski D. R. Mutations in direct repeat sequences and in a conserved sequence adjacent to the repeats result in a defective replication origin in plasmid R6K. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1480–1484. doi: 10.1073/pnas.82.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechulam Y., Fayat G., Blanquet S. Sequence of the Escherichia coli pheST operon and identification of the himA gene. J Bacteriol. 1985 Aug;163(2):787–791. doi: 10.1128/jb.163.2.787-791.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensa-Wilmot K., Carroll K., McMacken R. Transcriptional activation of bacteriophage lambda DNA replication in vitro: regulatory role of histone-like protein HU of Escherichia coli. EMBO J. 1989 Aug;8(8):2393–2402. doi: 10.1002/j.1460-2075.1989.tb08369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H. I., Mozola M. A., Friedman D. I. int-h: An int mutation of phage lambda that enhances site-specific recombination. Cell. 1980 Jul;20(3):721–729. doi: 10.1016/0092-8674(80)90318-9. [DOI] [PubMed] [Google Scholar]
- Miller H. I., Nash H. A. Direct role of the himA gene product in phage lambda integration. Nature. 1981 Apr 9;290(5806):523–526. doi: 10.1038/290523a0. [DOI] [PubMed] [Google Scholar]
- Miller H. I. Primary structure of the himA gene of Escherichia coli: homology with DNA-binding protein HU and association with the phenylalanyl-tRNA synthetase operon. Cold Spring Harb Symp Quant Biol. 1984;49:691–698. doi: 10.1101/sqb.1984.049.01.078. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Erickson H., Bastia D. Enhancer-origin interaction in plasmid R6K involves a DNA loop mediated by initiator protein. Cell. 1988 Feb 12;52(3):375–383. doi: 10.1016/s0092-8674(88)80030-8. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Patel I., Bastia D. Conformational changes in a replication origin induced by an initiator protein. Cell. 1985 Nov;43(1):189–197. doi: 10.1016/0092-8674(85)90023-6. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P., Filutowicz M., Helinski D. R. Replication from one of the three origins of the plasmid R6K requires coupled expression of two plasmid-encoded proteins. J Biol Chem. 1986 Jul 15;261(20):9534–9539. [PubMed] [Google Scholar]
- Peacock S., Weissbach H., Nash H. A. In vitro regulation of phage lambda cII gene expression by Escherichia coli integration host factor. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6009–6013. doi: 10.1073/pnas.81.19.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Chandler M., Galas D. J. Escherichia coli integration host factor bends the DNA at the ends of IS1 and in an insertion hotspot with multiple IHF binding sites. EMBO J. 1987 Aug;6(8):2479–2487. doi: 10.1002/j.1460-2075.1987.tb02529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C. A., Nash H. A. Bending of the bacteriophage lambda attachment site by Escherichia coli integration host factor. J Biol Chem. 1988 Mar 15;263(8):3554–3557. [PubMed] [Google Scholar]
- Shafferman A., Flashner Y., Hertman I., Lion M. Identification and characterization of the functional alpha origin of DNA replication of the R6K plasmid and its relatedness to the R6K beta and gamma origins. Mol Gen Genet. 1987 Jun;208(1-2):263–270. doi: 10.1007/BF00330452. [DOI] [PubMed] [Google Scholar]
- Shafferman A., Helinski D. R. Structural properties of the beta origin of replication of plasmid R6K. J Biol Chem. 1983 Apr 10;258(7):4083–4090. [PubMed] [Google Scholar]
- Snyder U. K., Thompson J. F., Landy A. Phasing of protein-induced DNA bends in a recombination complex. Nature. 1989 Sep 21;341(6239):255–257. doi: 10.1038/341255a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stalker D. M., Filutowicz M., Helinski D. R. Release of initiation control by a mutational alteration in the R6K pi protein required for plasmid DNA replication. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5500–5504. doi: 10.1073/pnas.80.18.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalker D. M., Kolter R., Helinski D. R. Nucleotide sequence of the region of an origin of replication of the antibiotic resistance plasmid R6K. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1150–1154. doi: 10.1073/pnas.76.3.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalker D. M., Kolter R., Helinski D. R. Plasmid R6K DNA replication. I. Complete nucleotide sequence of an autonomously replicating segment. J Mol Biol. 1982 Oct 15;161(1):33–43. doi: 10.1016/0022-2836(82)90276-5. [DOI] [PubMed] [Google Scholar]
- Stenzel T. T., Patel P., Bastia D. The integration host factor of Escherichia coli binds to bent DNA at the origin of replication of the plasmid pSC101. Cell. 1987 Jun 5;49(5):709–717. doi: 10.1016/0092-8674(87)90547-2. [DOI] [PubMed] [Google Scholar]
- Thompson J. F., Landy A. Empirical estimation of protein-induced DNA bending angles: applications to lambda site-specific recombination complexes. Nucleic Acids Res. 1988 Oct 25;16(20):9687–9705. doi: 10.1093/nar/16.20.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R. K., Miller J. L., Miller H. I., Friedman D. I., Howe M. M. Isolation and mapping of Mu nu mutants which grow in him mutants of E. coli. Virology. 1982 Jul 15;120(1):269–272. doi: 10.1016/0042-6822(82)90027-7. [DOI] [PubMed] [Google Scholar]