Abstract
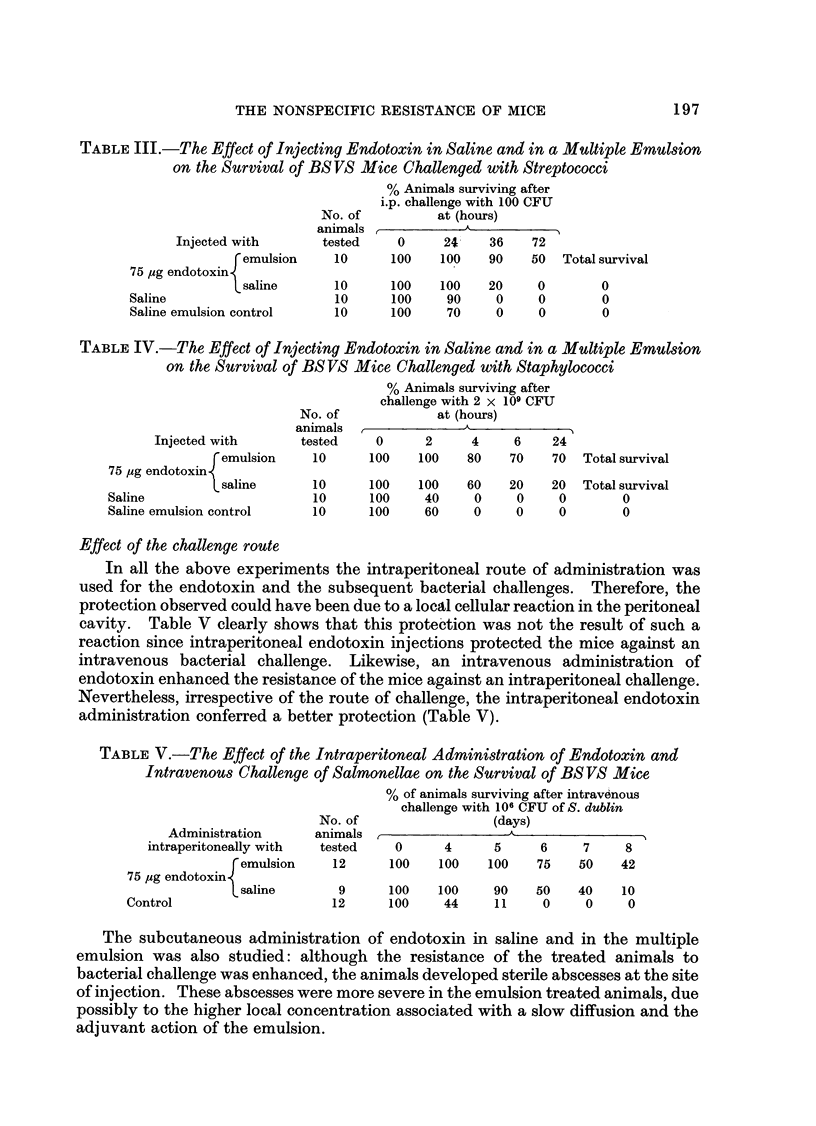
The nonspecific resistance of mice to challenge was enhanced following the administration of an E. coli O55 B5 endotoxin. Although the route of administration of the endotoxin and the challenge organism were varied, the nonspecific resistance of the animal was enhanced in all the experiments. The efficiency of this resistance was highest when the inducing substance and the challenge dose of bacteria were administered intraperitoneally. Poly I: C and double stranded RNA were also studied but were much less effective than endotoxin in stimulating a resistance to infection.
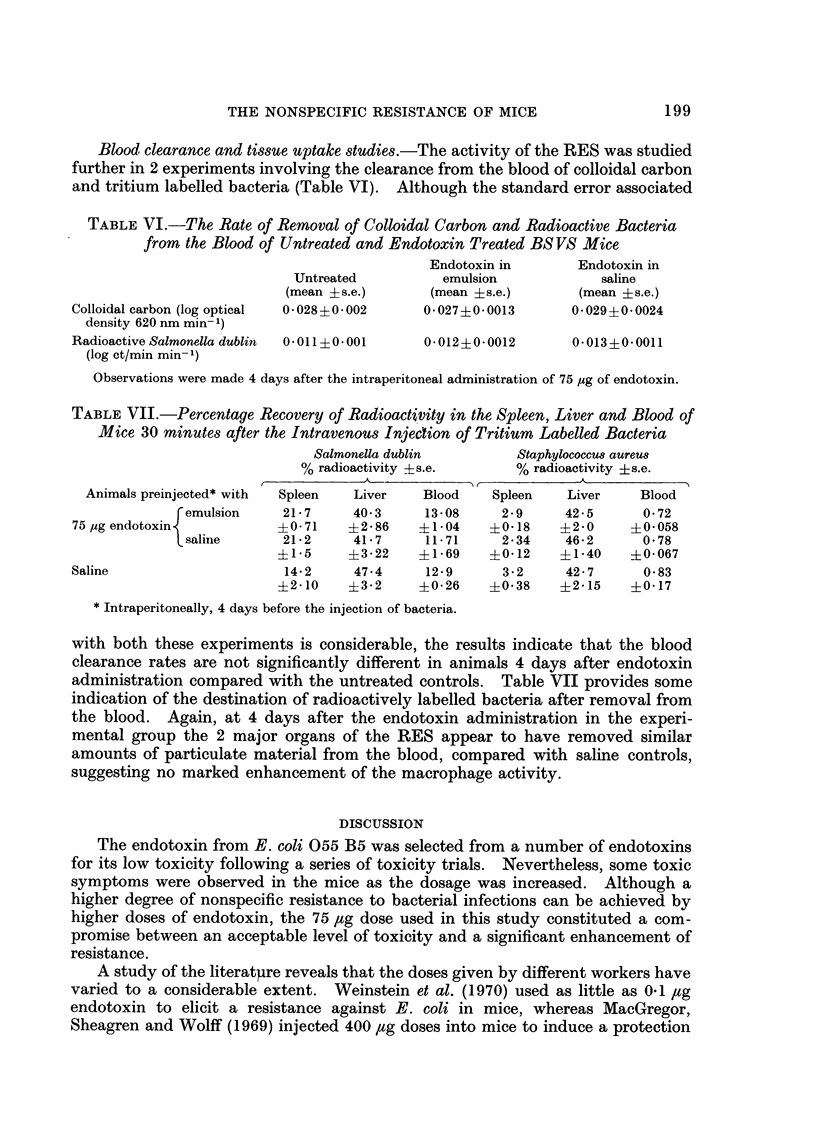
Stimulation of the fixed macrophages could not explain fully the enhanced resistance, since the clearance rates of colloidal carbon and radioactively labelled bacteria from the blood were not significantly enhanced after the administration of endotoxin. Furthermore, splenectomized animals, and animals injected with agents which interfere with the RES activity, trypan blue and corticosteroids, still developed a degree of nonspecific resistance to infection.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIOZZI G., BENACERRAF B., HALPERN B. N. Quantitative study of the granulopectic activity of the reticulo-endothelial system. II. A study of the kinetics of the R. E. S. in relation to the dose of carbon injected; relationship between the weight of the organs and their activity. Br J Exp Pathol. 1953 Aug;34(4):441–457. [PMC free article] [PubMed] [Google Scholar]
- BIOZZI G., BENACERRAF B., HALPERN B. N. The effect of Salm. typhi and its endotoxin on the phagocytic activity of the reticuloendothelial system in mice. Br J Exp Pathol. 1955 Jun;36(3):226–235. [PMC free article] [PubMed] [Google Scholar]
- Brownlie J., Hibbitt K. G. Antimicrobial proteins isolated from bovine cervical mucus. J Reprod Fertil. 1972 Jun;29(3):337–347. doi: 10.1530/jrf.0.0290337. [DOI] [PubMed] [Google Scholar]
- DUBOS R. J., SCHAEDLER R. W. Reversible changes in the susceptibility of mice to bacterial infections. I. Changes brought about by injection of pertussis vaccine or of bacterial endotoxins. J Exp Med. 1956 Jul 1;104(1):53–65. doi: 10.1084/jem.104.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. K., Tytell A. A., Lampson G. P., Hilleman M. R. Cellular control of interferon production and release after treatment with poly I:C inducer. Proc Soc Exp Biol Med. 1972 Jun;140(2):710–714. doi: 10.3181/00379727-140-36537. [DOI] [PubMed] [Google Scholar]
- Gladstone G. P., Walton E. The effect of iron and haematin on the killing of staphylococci by rabbit polymorphs. Br J Exp Pathol. 1971 Oct;52(5):452–464. [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G. Bactericidal action of histone. J Exp Med. 1958 Dec 1;108(6):925–944. doi: 10.1084/jem.108.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HO M. INTERFERON-LIKE VIRAL INHIBITOR IN RABBITS AFTER INTRAVENOUS ADMINISTRATION OF ENDOTOXIN. Science. 1964 Dec 11;146(3650):1472–1474. doi: 10.1126/science.146.3650.1472. [DOI] [PubMed] [Google Scholar]
- HOWARD J. G., ROWLEY D., WARDLAW A. C. Investigations on the mechanism of stimulation of non-specific immunity by bacterial lipopolysaccharides. Immunology. 1958 Jul;1(3):181–203. [PMC free article] [PubMed] [Google Scholar]
- Herbert W. J. Multiple emulsions. A new form of mineral-oil antigen adjuvant. Lancet. 1965 Oct 16;2(7416):771–771. doi: 10.1016/s0140-6736(65)90816-0. [DOI] [PubMed] [Google Scholar]
- Hibbitt K. G., Cole C. B., Reiter B. Antimicrobial proteins isolated from the teat canal of the cow. J Gen Microbiol. 1969 Jun;56(3):365–371. doi: 10.1099/00221287-56-3-365. [DOI] [PubMed] [Google Scholar]
- Jahiel R. I., Vilcek J., Nussenzweig R., Vanderberg J. Interferon inducers protect mice against plasmodium berghei malaria. Science. 1968 Aug 23;161(3843):802–804. doi: 10.1126/science.161.3843.802. [DOI] [PubMed] [Google Scholar]
- MacGregor R. R., Sheagren J. N., Wolff S. M. Endotoxin-induced modification of Plasmodium berghei infection in mice. J Immunol. 1969 Jan;102(1):131–139. [PubMed] [Google Scholar]
- Mills L. C. Corticosteroids in endotoxic shock. Proc Soc Exp Biol Med. 1971 Nov;138(2):507–511. doi: 10.3181/00379727-138-35928. [DOI] [PubMed] [Google Scholar]
- Morahan P. S., Munson A. E., Regelson W., Commerford S. L., Hamilton L. D. Antiviral activity and side effects of polyriboinosinic-cytidylic acid complexes as affected by molecular size. Proc Natl Acad Sci U S A. 1972 Apr;69(4):842–846. doi: 10.1073/pnas.69.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D. Stimulation of natural immunity to Escherichia coli infection: observations on mice. Lancet. 1955 Jan 29;268(6857):232–234. doi: 10.1016/s0140-6736(55)90163-x. [DOI] [PubMed] [Google Scholar]
- Remington J. S., Merigan T. C. Interferon: protection of cells infected with an intracellular protozoan (Toxoplasma gondii). Science. 1968 Aug 23;161(3843):804–806. doi: 10.1126/science.161.3843.804. [DOI] [PubMed] [Google Scholar]
- Tew J. G., Scott R. L., Donaldson D. M. Plasma beta-lysin and lysozyme following endotoxin administration and the generalized Shwartzman reaction. Proc Soc Exp Biol Med. 1971 Feb;136(2):473–478. doi: 10.3181/00379727-136-35291. [DOI] [PubMed] [Google Scholar]
- WAGNER R. R., SNYDER R. M., HOOK E. W., LUTTRELL C. N. Effect of bacterial endotoxin on resistance of mice to viral encephalitides; including comparative studies of the interference phenomenon. J Immunol. 1959 Jul;83(1):87–98. [PubMed] [Google Scholar]
- Weinstein M. J., Waitz J. A., Came P. E. Induction of resistance to bacterial infections of mice with poly I-poly C. Nature. 1970 Apr 11;226(5241):170–170. doi: 10.1038/226170a0. [DOI] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. I. Resolution of antibacterial and enzymatic activities. J Bacteriol. 1966 Feb;91(2):750–754. doi: 10.1128/jb.91.2.750-754.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


