Abstract
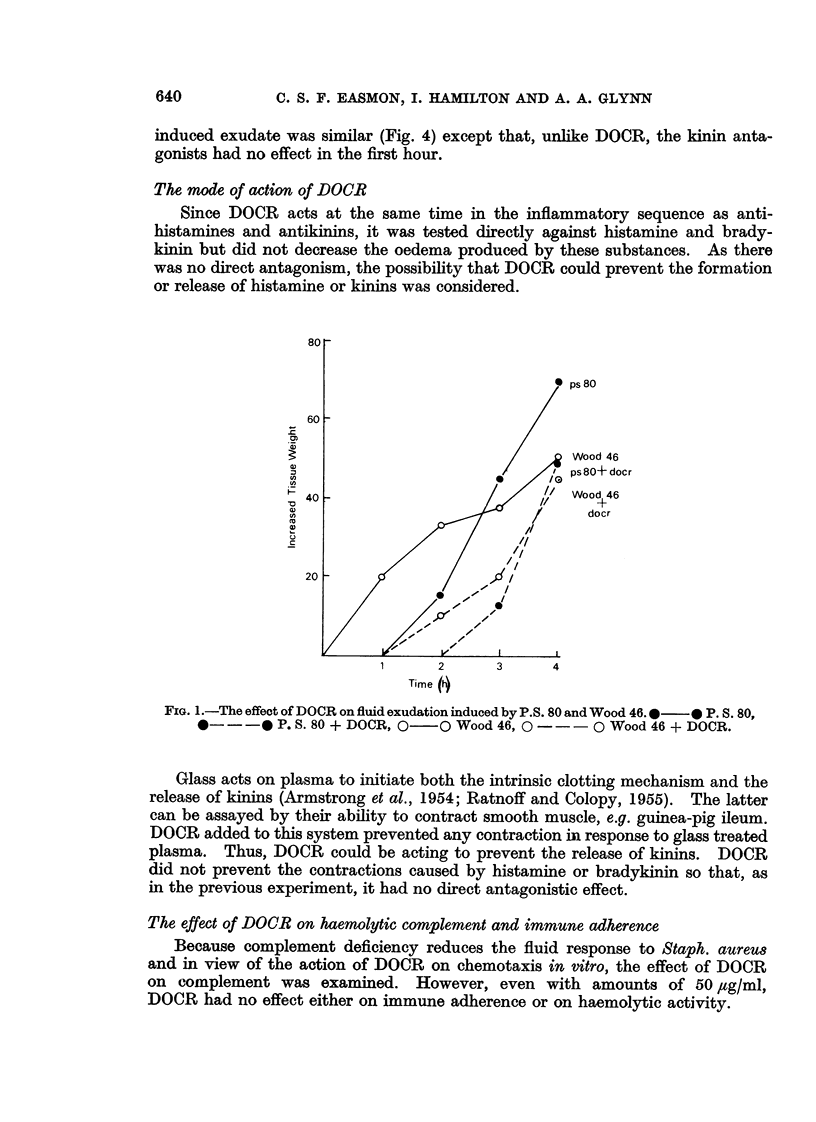
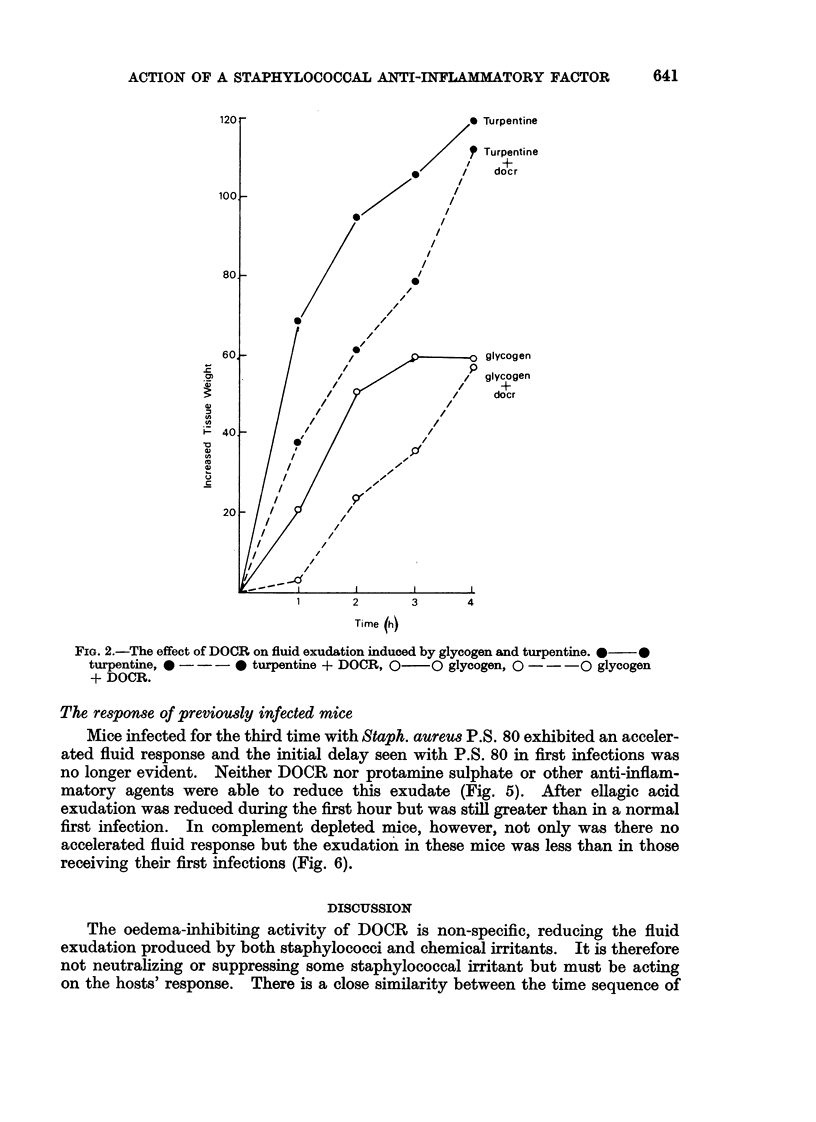
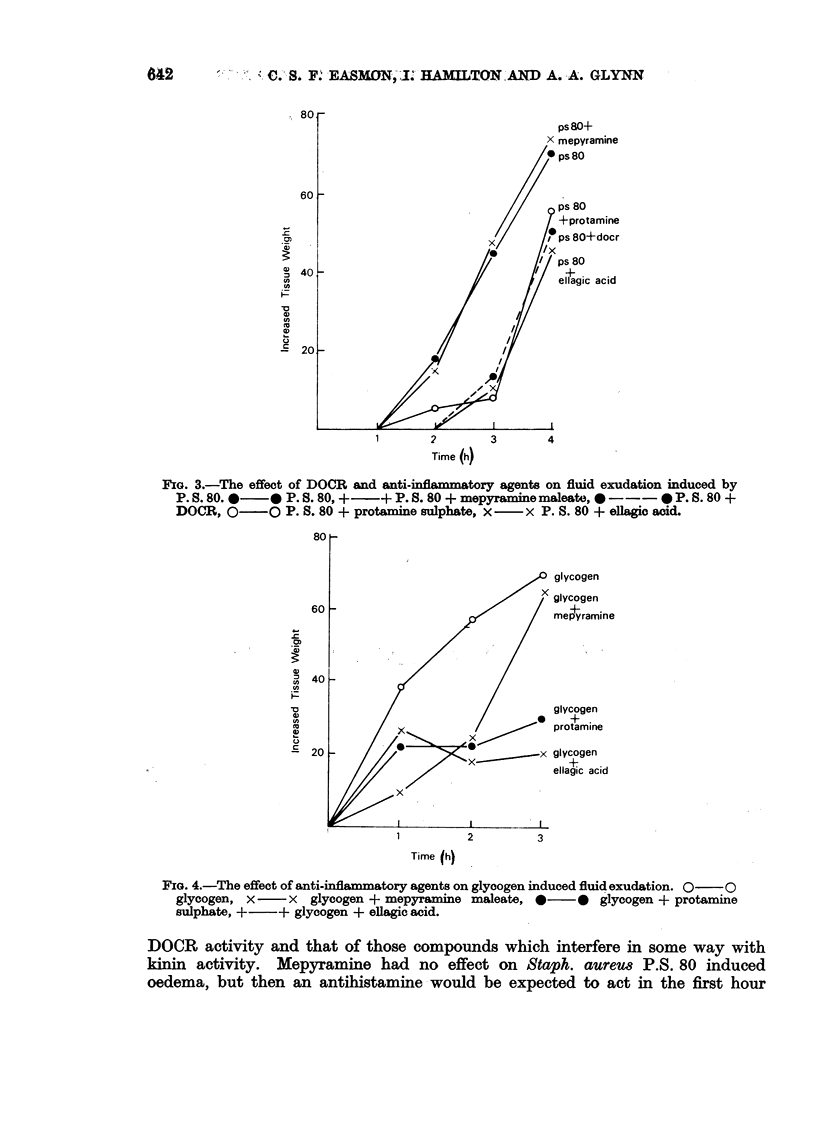
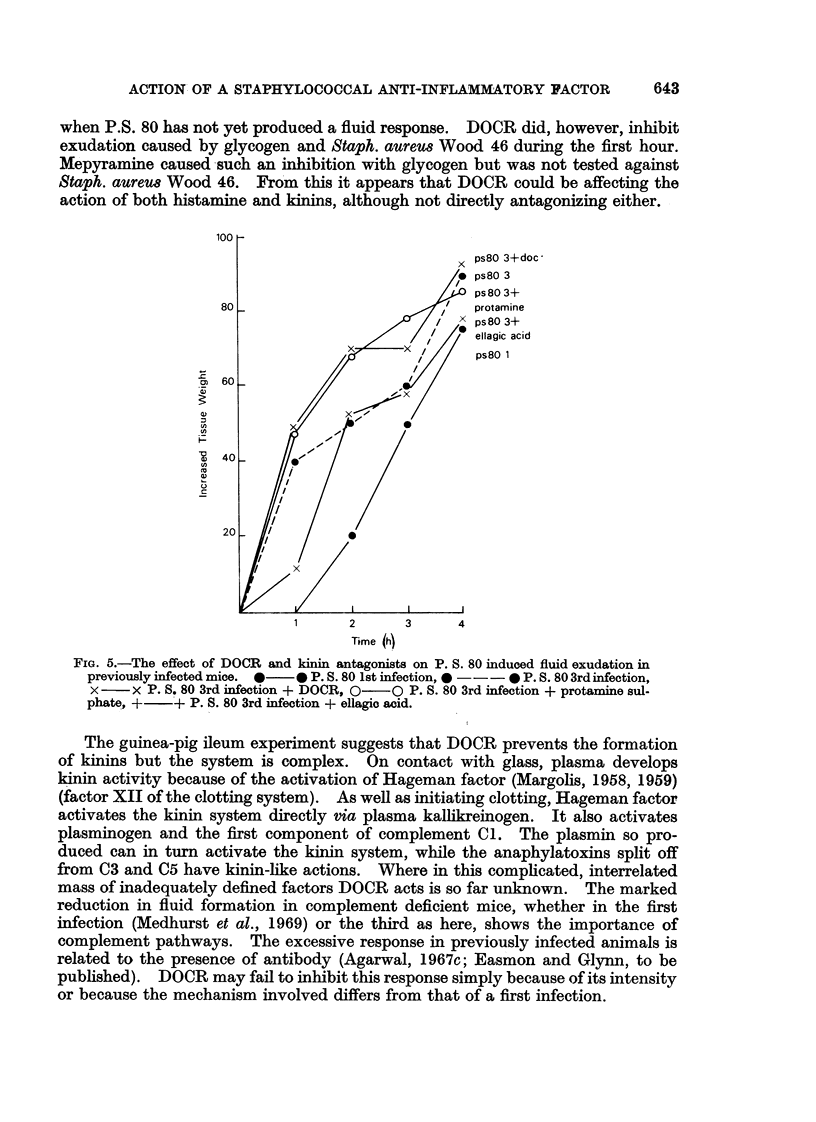
A mucopeptide protein residue, DOCR, is an impedin, isolated from virulent strains of Staphylococcus aureus, which has been shown previously to potentiate staphylococcal infection by inhibiting the early phase of the inflammatory response. Comparison of the effects of DOCR with those of various pharmacological inhibitors on inflammation in vivo and on the guinea-pig ileum in vitro suggests that DOCR prevents the release of kinins by acting somewhere in the Hageman factor-kinin pathway. DOCR did not prevent the more massive fluid exudate which occurred in previously infected animals. It had no effect in vitro on the haemolytic activity of complement or on immune adhesion.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal D. S. Subcutaneous staphylococcal infection in mice. 3. Effect of active and passive immunization and anti-inflammatory drugs. Br J Exp Pathol. 1967 Oct;48(5):483–500. [PMC free article] [PubMed] [Google Scholar]
- Agarwal D. S. Subcutaneous staphylococcal infection in mice. I. The role of cotton-dust in enhancing infection. Br J Exp Pathol. 1967 Aug;48(4):436–449. [PMC free article] [PubMed] [Google Scholar]
- Agarwal D. S. Subcutaneous staphylococcal infection in mice. II. The inflammatory response to different strains of staphylococci and micrococci. Br J Exp Pathol. 1967 Oct;48(5):468–482. [PMC free article] [PubMed] [Google Scholar]
- BURKE J. F., MILES A. A. The sequence of vascular events in early infective inflammation. J Pathol Bacteriol. 1958 Jul;76(1):1–19. [PubMed] [Google Scholar]
- Di Rosa M., Giroud J. P., Willoughby D. A. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971 May;104(1):15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- MARGOLIS J. Activation of a permeability factor in plasma by contact with glass. Nature. 1958 Mar 1;181(4609):635–636. doi: 10.1038/181635a0. [DOI] [PubMed] [Google Scholar]
- MARGOLIS J. Hagerman factor and capillary permeability. Aust J Exp Biol Med Sci. 1959 Jun;37:239–244. doi: 10.1038/icb.1959.24. [DOI] [PubMed] [Google Scholar]
- MILES A. A., MILES E. M., BURKE J. The value and duration of defence reactions of the skin to the primary lodgement of bacteria. Br J Exp Pathol. 1957 Feb;38(1):79–96. [PMC free article] [PubMed] [Google Scholar]
- Medhurst F. A., Hill M. J., Glynn A. A. The effect of antilymphocyte serum on subcutaneous staphylococcal infections in normal, immune and complement-deficient mice. J Med Microbiol. 1969 May;2(2):147–159. doi: 10.1099/00222615-2-2-147. [DOI] [PubMed] [Google Scholar]
- NISHIOKA K. Measurements of complement by agglutination of human erythrocytes reacting in immune-adherence. J Immunol. 1963 Jan;90:86–97. [PubMed] [Google Scholar]
- Noble W. C. The production of subcutaneous staphylococcal skin lesions in mice. Br J Exp Pathol. 1965 Jun;46(3):254–262. [PMC free article] [PubMed] [Google Scholar]
- RATNOFF O. D., COLOPY J. E. A familial hemorrhagic trait associated with a deficiency of a clot-promoting fraction of plasma. J Clin Invest. 1955 Apr;34(4):602–613. doi: 10.1172/JCI103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBERG L. T., TACHIBANA D. K. Activity of mouse complement. J Immunol. 1962 Dec;89:861–867. [PubMed] [Google Scholar]
- WARD P. A., COCHRANE C. G. BOUND COMPLEMENT AND IMMUNOLOGIC INJURY OF BLOOD VESSELS. J Exp Med. 1965 Feb 1;121:215–234. doi: 10.1084/jem.121.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B. B., Hill M. J. Inhibition of leukocyte migration by a staphylococcal factor. J Bacteriol. 1969 Jun;98(3):1030–1035. doi: 10.1128/jb.98.3.1030-1035.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby D. A., Coote E., Turk J. L. Complement in acute inflammation. J Pathol. 1969 Feb;97(2):295–305. doi: 10.1002/path.1710970215. [DOI] [PubMed] [Google Scholar]


