Abstract
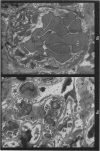
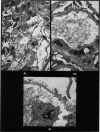
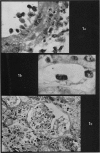
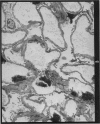
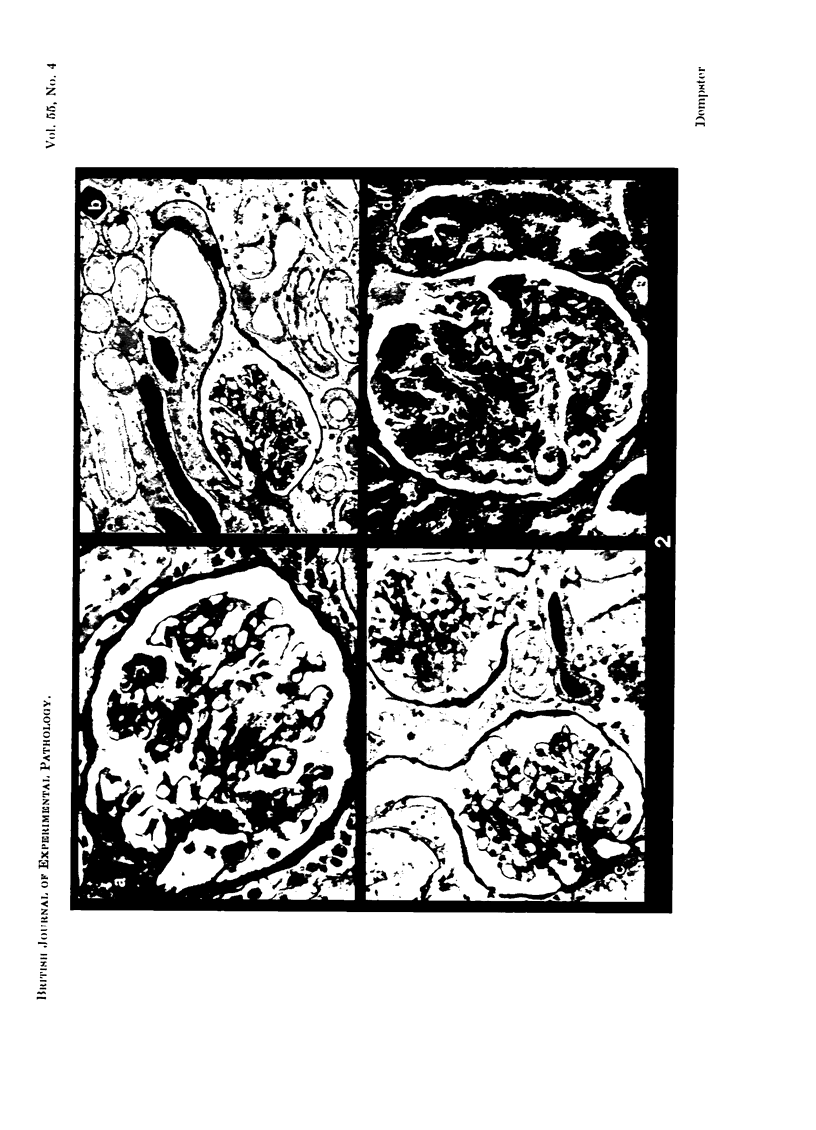
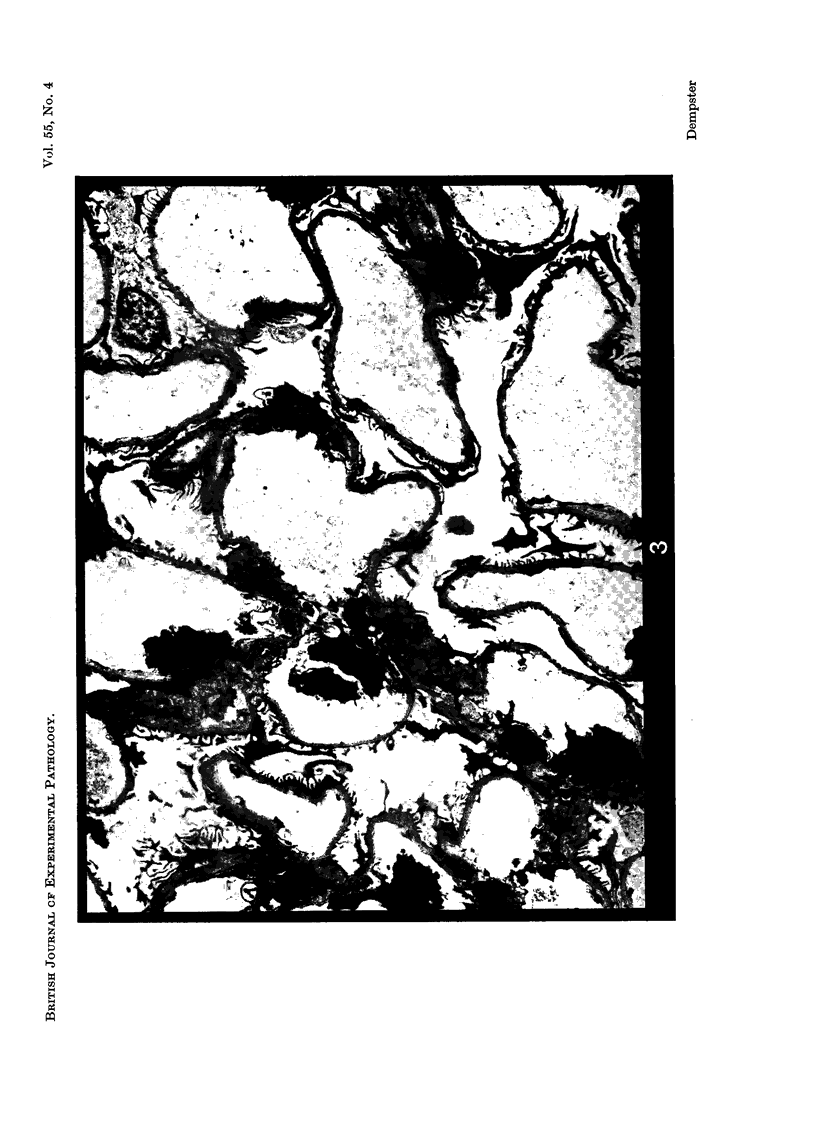
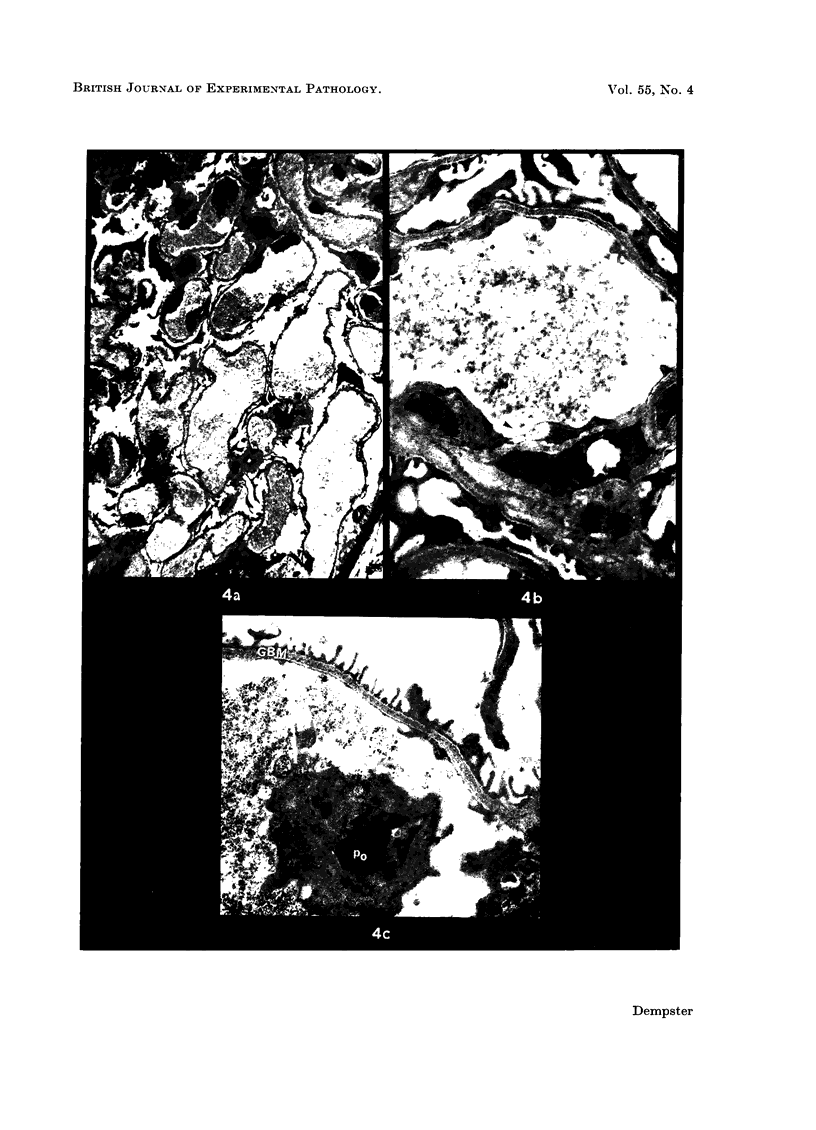
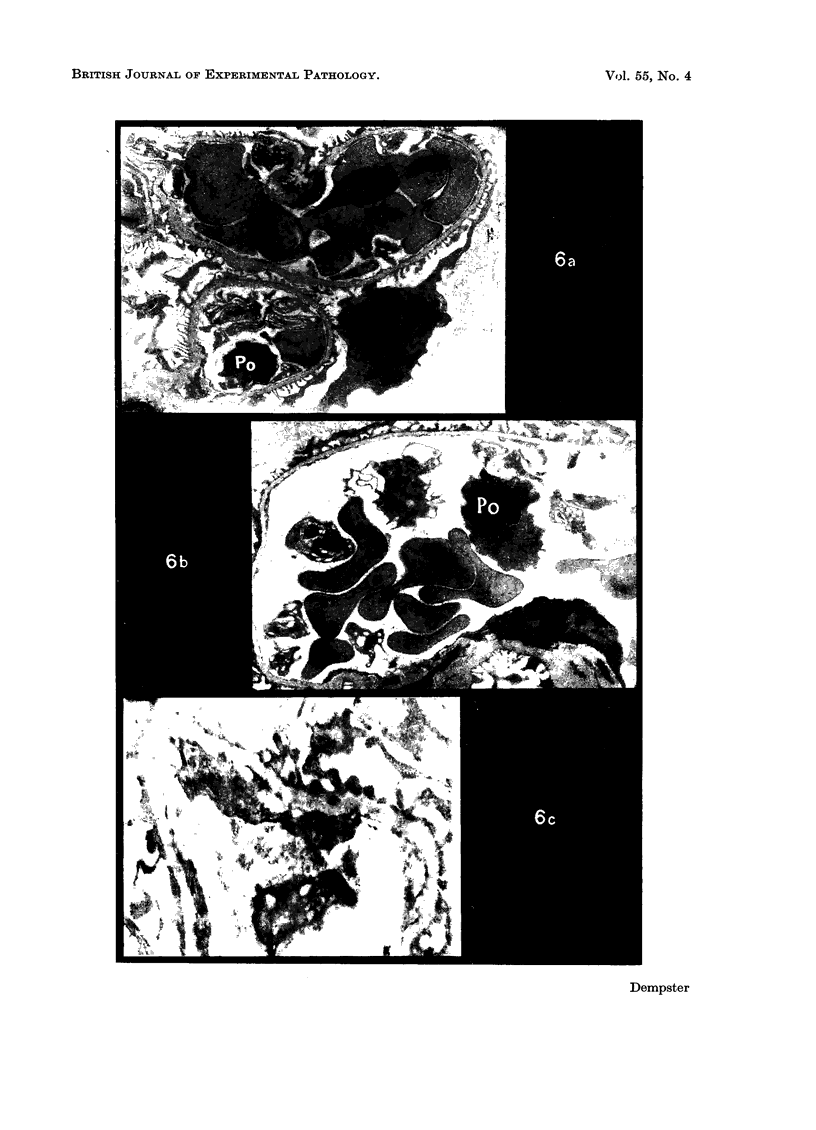
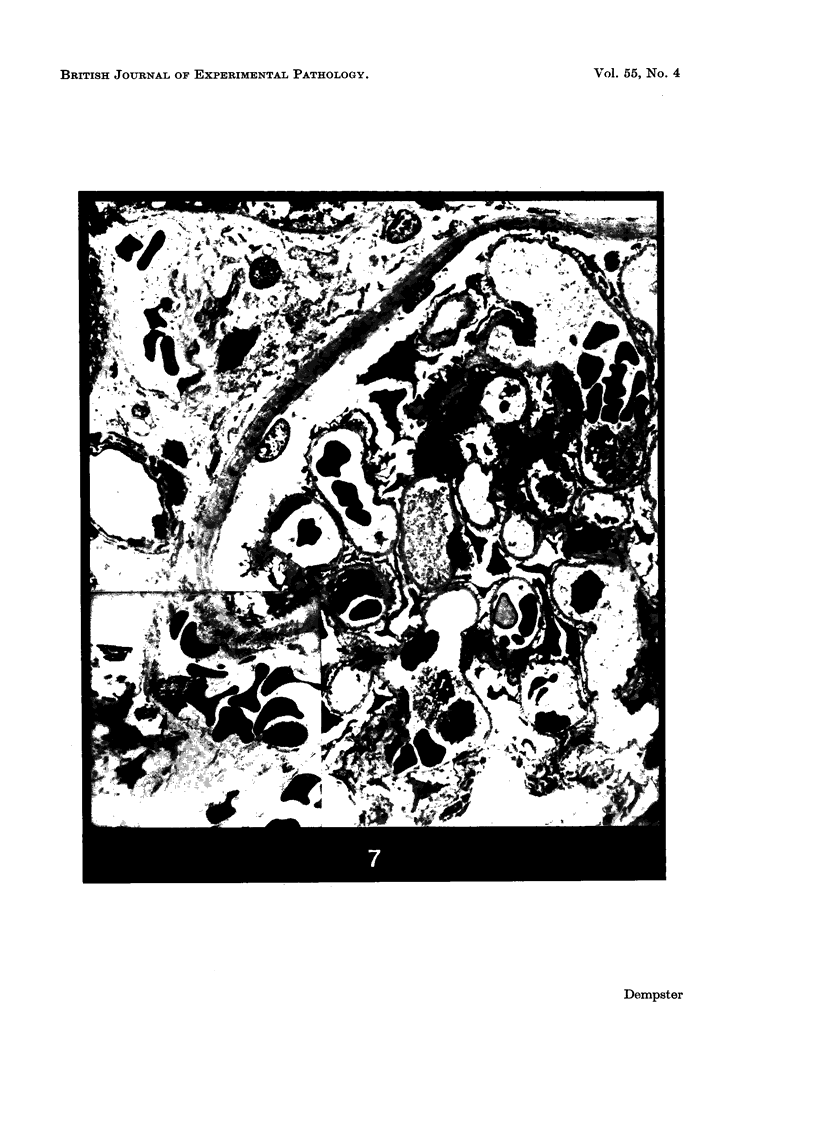
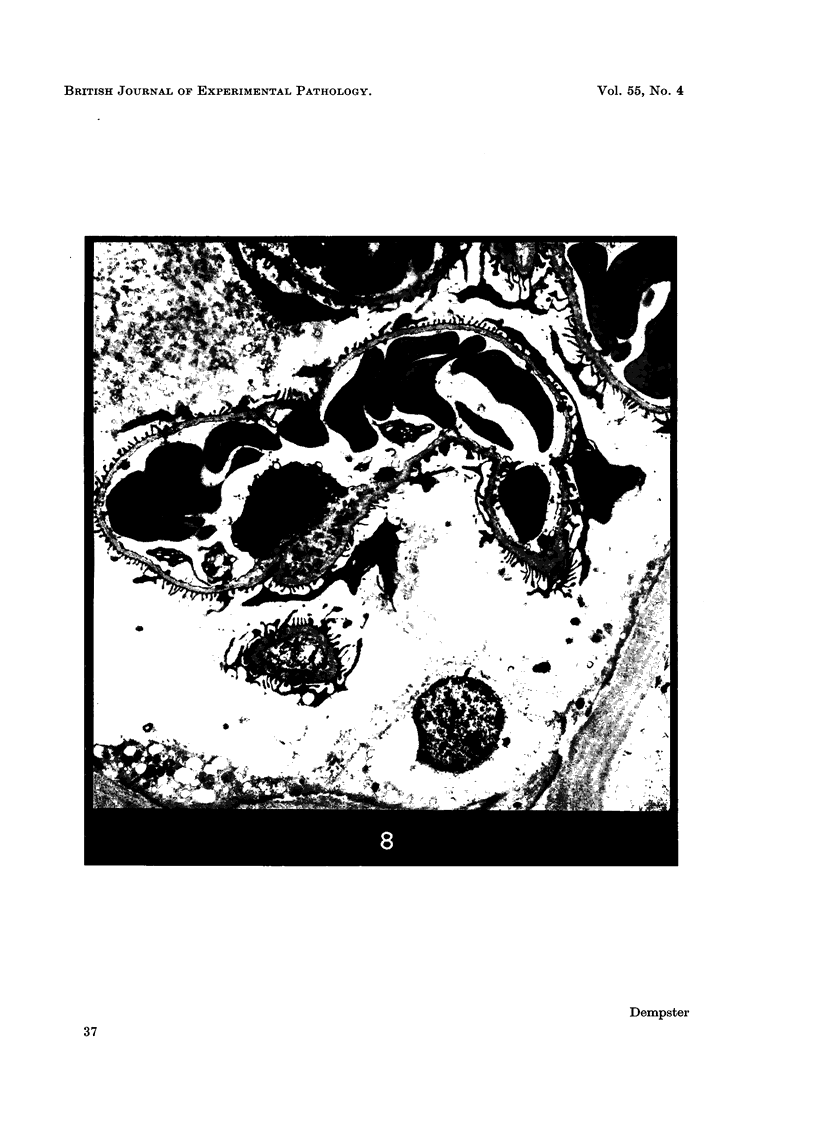
Recipients which have already rejected allografts of skin react rapidly and violently against a subsequently allotransplanted kidney. This is the renal allotransplant second-set reaction, the primary manifestation of which is a sudden severe afferent vasoconstriction. As a result of this vasomotor disturbance, red blood cells and platelets sometimes aggregate in the glomerular capillaries and the renal tubule cells suffer necrosis. The precipitating cause of the vasomotor disturbance has not been determined but platelet aggregation can be ruled out since in ancrod (Arvin)-treated recipients this does not occur although a second-set reaction develops. No evidence of glomerular basement membrane damage, due either to anti-glomerular basement membrane antibodies or antigen antibody complexes, was detected by the current ultrastructural studies. Since in these experiments the recipients were previously sensitized by skin allografts, a cytotoxic factor which is directed against specific renal components should not be expected to mediate the renal allotransplant second-set reaction. There is, at some structural level, common antigenicity between skin, heart and kidney and, since all three tissues succumb to changes in their vascular systems, it suggests some common antigenic factor in blood vessels. The features of the renal second-set reaction have been compared with other established renal immune disease states but none can compare in rapidity and severity of action with the second-set reaction.
Full text
PDF


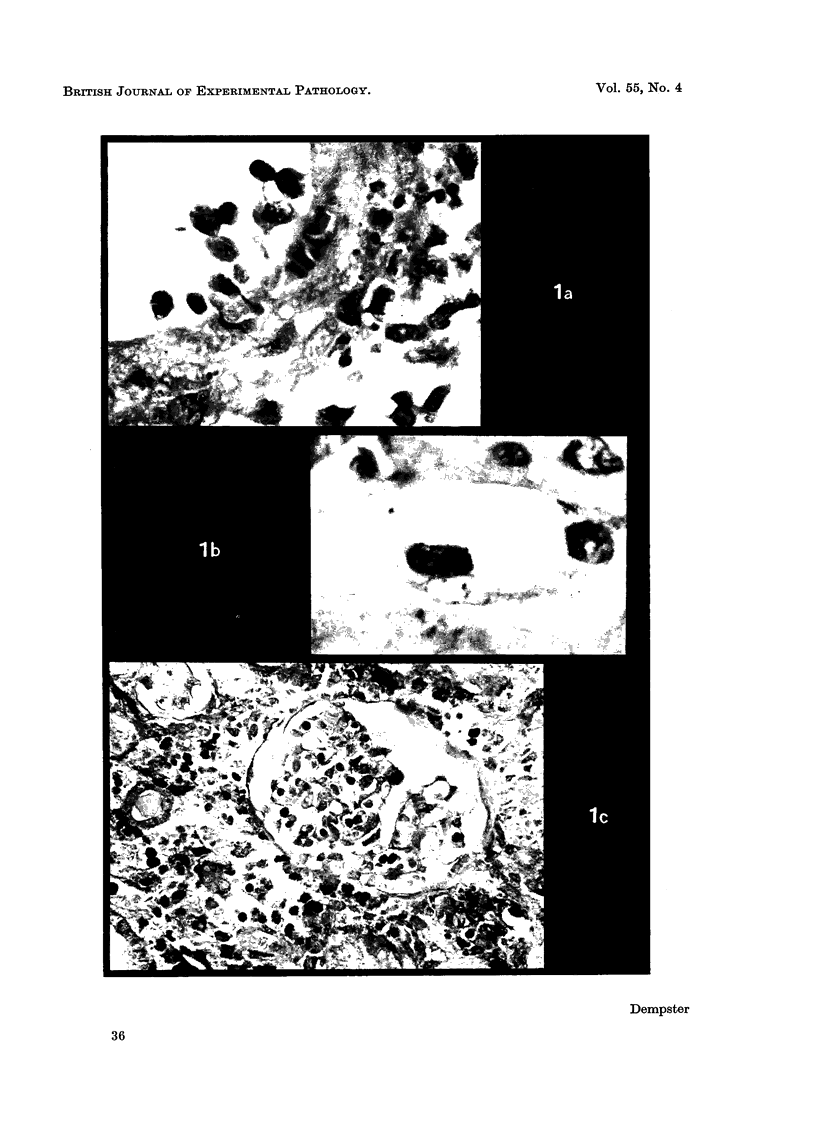













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahrons S., Lund B. Renal transplantation in rabbits. 8. The relationship between lymphocytotoxic antibodies, renal IgG deposits and the renal allograft reaction in rabbits sensitized by multiple skin grafts. Tissue Antigens. 1972;2(2):112–122. doi: 10.1111/j.1399-0039.1972.tb00125.x. [DOI] [PubMed] [Google Scholar]
- Bellanti J. A., Green R. E. Immunological reactivity. Expression of efficiency in elimination of foreignness. Lancet. 1971 Sep 4;2(7723):526–529. doi: 10.1016/s0140-6736(71)90443-0. [DOI] [PubMed] [Google Scholar]
- Chase W. H., Paine L. D., Lamoureux G., Taylor H. E. Ultrastructural study of the glomerulonephritis produced by antilymphocyte globulin in monkeys. Lab Invest. 1972 Oct;27(4):393–399. [PubMed] [Google Scholar]
- Comparative trial of surgery and radiotherapy for the primary treatment of small-celled or oat-celled carcinoma of the bronchus. First report to the Medical Research Council by the working-party on the evaluation of different methods of therapy in carcinoma of the bronchus. Lancet. 1966 Nov 5;2(7471):979–986. [PubMed] [Google Scholar]
- DEMPSTER W. J. A consideration of the cause of functional arrest of homotransplanted kidneys. Br J Urol. 1955 Mar;27(1):66–86. doi: 10.1111/j.1464-410x.1955.tb03445.x. [DOI] [PubMed] [Google Scholar]
- Dempster W. J., Brown D. L. The nature of experimental second-set kidney transplant rejection. 3. The role of complement. Br J Exp Pathol. 1971 Jun;52(3):280–291. [PMC free article] [PubMed] [Google Scholar]
- Dempster W. J. Functional aspects of the rejection of transplanted kidneys. Calif Med. 1971 Dec;115(6):17–39. [PMC free article] [PubMed] [Google Scholar]
- Dempster W. J. Hypertension and acute rejection processes in allotransplanted kidneys. Br J Exp Pathol. 1970 Apr;51(2):149–170. [PMC free article] [PubMed] [Google Scholar]
- Dempster W. J. The nature of experimental second-set kidney transplant rejection. 2. The mimicking of the haemodynamic upset by pharmacological and other means. Br J Exp Pathol. 1971 Apr;52(2):172–185. [PMC free article] [PubMed] [Google Scholar]
- Ferrone S., Cooper N. R., Pellegrino M. A., Reisfeld R. A. The lymphocytotoxic reaction: the mechanism of rabbit complement action. J Immunol. 1971 Oct;107(4):939–947. [PubMed] [Google Scholar]
- Fish A. J., Herdman R. C., Pkelly W. D., Good R. A. Glomerular changes in well-functioning human renal homografts. Transplantation. 1967 Sep 5;5(5):1338–1343. doi: 10.1097/00007890-196709000-00013. [DOI] [PubMed] [Google Scholar]
- Ford P. M. A naturally occurring human antibody to loops of Henle. Clin Exp Immunol. 1973 Aug;14(4):569–572. [PMC free article] [PubMed] [Google Scholar]
- Germuth F. G., Jr, Kelemen W. A., Pollack A. D. Immune complex disease. II. The role of circulatory dynamics and glomerular filtration in the development of experimental glomerulonephritis. Johns Hopkins Med J. 1967 Apr;120(4):252–261. [PubMed] [Google Scholar]
- HEYMANN W., HACKEL D. B., HARWOOD S., WILSON S. G., HUNTER J. L. Production of nephrotic syndrome in rats by Freund's adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med. 1959 Apr;100(4):660–664. doi: 10.3181/00379727-100-24736. [DOI] [PubMed] [Google Scholar]
- Honour A. J., Pickering G. W., Sheppard B. L. Ultrastructure and behaviour of platelet thrombi in injured arteries. Br J Exp Pathol. 1971 Oct;52(5):482–494. [PMC free article] [PubMed] [Google Scholar]
- Jokinen E. J., Wegelius O. Tubular and interstitial nephritis produced by homologous heart homogenate in rabbits. 2. Anti-heart antibodies. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(5):547–552. doi: 10.1111/j.1699-0463.1970.tb04339.x. [DOI] [PubMed] [Google Scholar]
- Kissmeyer-Nielsen F., Olsen S., Petersen V. P., Fjeldborg O. Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells. Lancet. 1966 Sep 24;2(7465):662–665. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- Klassen J., McCluskey R. T., Milgrom F. Nonglomerular renal disease produced in rabbits by immunization with homologous kidney. Am J Pathol. 1971 May;63(2):333–358. [PMC free article] [PubMed] [Google Scholar]
- LENNOX B., DEMPSTER W. J., BOAG J. W. Suppression of the tuberculin reaction in rabbits by total body irradiation with x-rays. Br J Exp Pathol. 1952 Aug;33(4):380–389. [PMC free article] [PubMed] [Google Scholar]
- Lambert P. B., Ming S. C., Palmerio C. Vasomotor factors in the Shwartzman phenomenon. Am J Pathol. 1969 Dec;57(3):559–580. [PMC free article] [PubMed] [Google Scholar]
- Latour J. G., Prejean J. B., Margaretten W. Corticosteroids and the generalized Shwartzman reaction. Mechanisms of sensitization in the rabbit. Am J Pathol. 1971 Oct;65(1):189–202. [PMC free article] [PubMed] [Google Scholar]
- MacDonald A. S., Bell W. R., Busch G. J., Ghose T., Chan C. C., Falvey C. F., Merrill J. P. A comparison of hyperacute canine renal allograft and sheep-to-dog xenograft rejection. Transplantation. 1972 Feb;13(2):146–154. doi: 10.1097/00007890-197202000-00015. [DOI] [PubMed] [Google Scholar]
- Manaligod J. R., Krakower C. A., Greenspon S. A. Glomerular changes induced by extrarenal foci of inflammation and by polymorphonuclear cell lysates. Am J Pathol. 1969 Sep;56(3):533–551. [PMC free article] [PubMed] [Google Scholar]
- McConnell I. Antigen receptors on the surface of antibody-secreting cells. Nat New Biol. 1971 Oct 6;233(40):177–179. doi: 10.1038/newbio233177a0. [DOI] [PubMed] [Google Scholar]
- Milgrom F., Litvak B. I., Kano K., Witebsky E. Humoral antibodies in renal homograft. JAMA. 1966 Oct 17;198(3):226–230. [PubMed] [Google Scholar]
- PAPPAS G. D., ROSS M. H., THOMAS L. Studies on the generalized Shwartzman reaction. VIII. The appearance, by electron microscopy, of intravascular fibrinoid in the glomerular capillaries during the reaction. J Exp Med. 1958 Mar 1;107(3):333–340. doi: 10.1084/jem.107.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palutke M., Kihn R., Perry M., Riddle J., Rector F., Rosenberg J. C. Role of leukocytes and lysosomal enzymes in antibody-mediated (hyperacute) rejection of renal heterografts. Lab Invest. 1972 Sep;27(3):287–295. [PubMed] [Google Scholar]
- Rosen S. M., Truniger B. P., Kriek H. R., Murray J. E., Merrill J. P. Intrarenal distribution of blood flow in the transplanted dog kidney: effect of denervation and rejection. J Clin Invest. 1967 Jul;46(7):1239–1253. doi: 10.1172/JCI105617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma H. M., Moore S., Merrick H. W., Smith M. R. Platelets in early hyperacute allograft rejection in kidneys and their modification by sulfinpyrazone (Anturan) therapy. An experimental study. Am J Pathol. 1972 Mar;66(3):445–460. [PMC free article] [PubMed] [Google Scholar]
- Shigematsu H. Glomerular events during the initial phase of rat Masugi nephritis. Virchows Arch B Cell Pathol. 1970;5(3):187–200. [PubMed] [Google Scholar]
- Shigematsu H., Shishido H., Kuhara K., Tsuchida H., Suzuki H. Participation of monocytes in transient glomerular hypercellularity in poststreptococcal glomerulonephritis. Virchows Arch B Cell Pathol. 1973 Mar 30;12(4):367–370. doi: 10.1007/BF02894013. [DOI] [PubMed] [Google Scholar]
- Weir D. M. The immunologicial consequences of cell death. Lancet. 1967 Nov 18;2(7525):1071–1073. doi: 10.1016/s0140-6736(67)90342-x. [DOI] [PubMed] [Google Scholar]
- Westberg N. G., Michael A. F. Immunohistopathology of diabetic glomerulosclerosis. Diabetes. 1972 Mar;21(3):163–174. doi: 10.2337/diab.21.3.163. [DOI] [PubMed] [Google Scholar]