Abstract
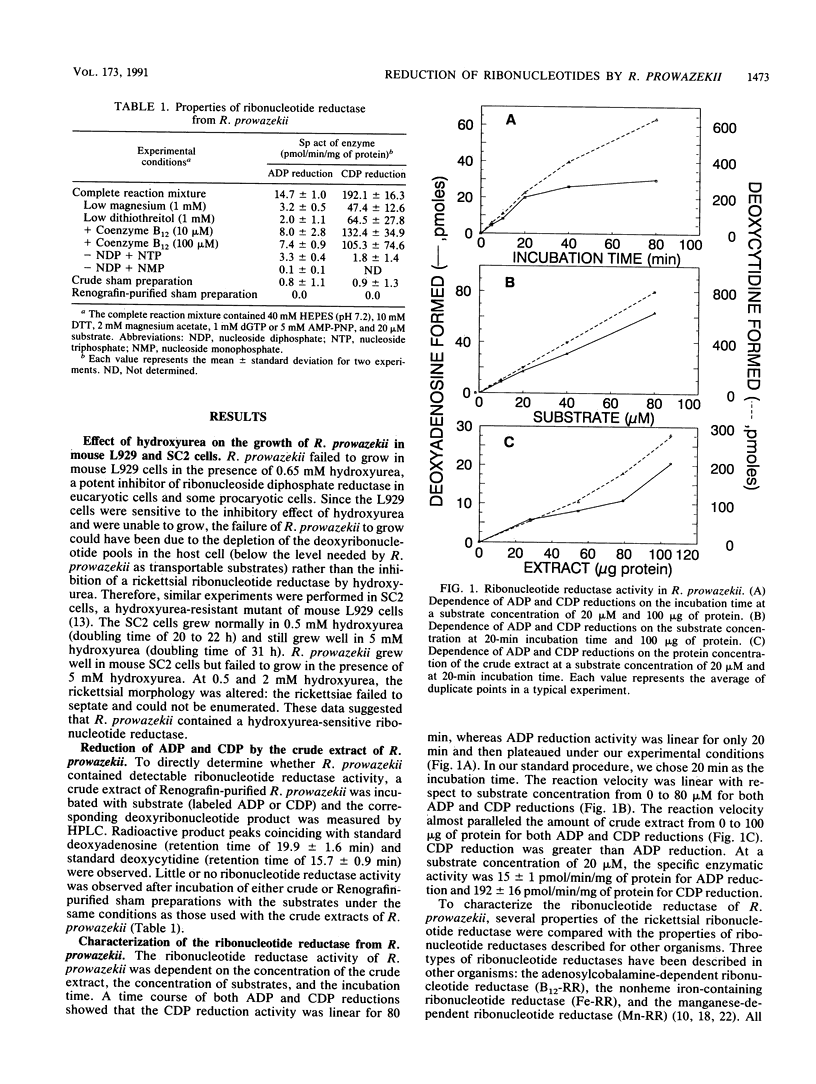
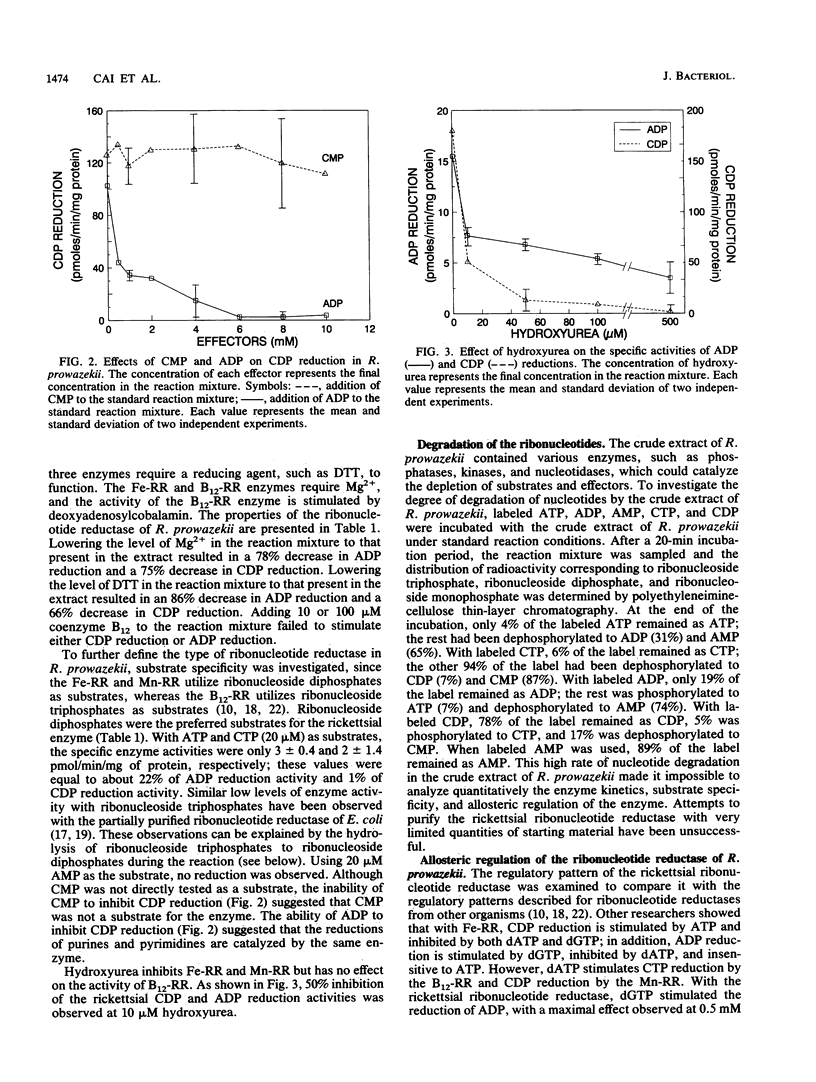
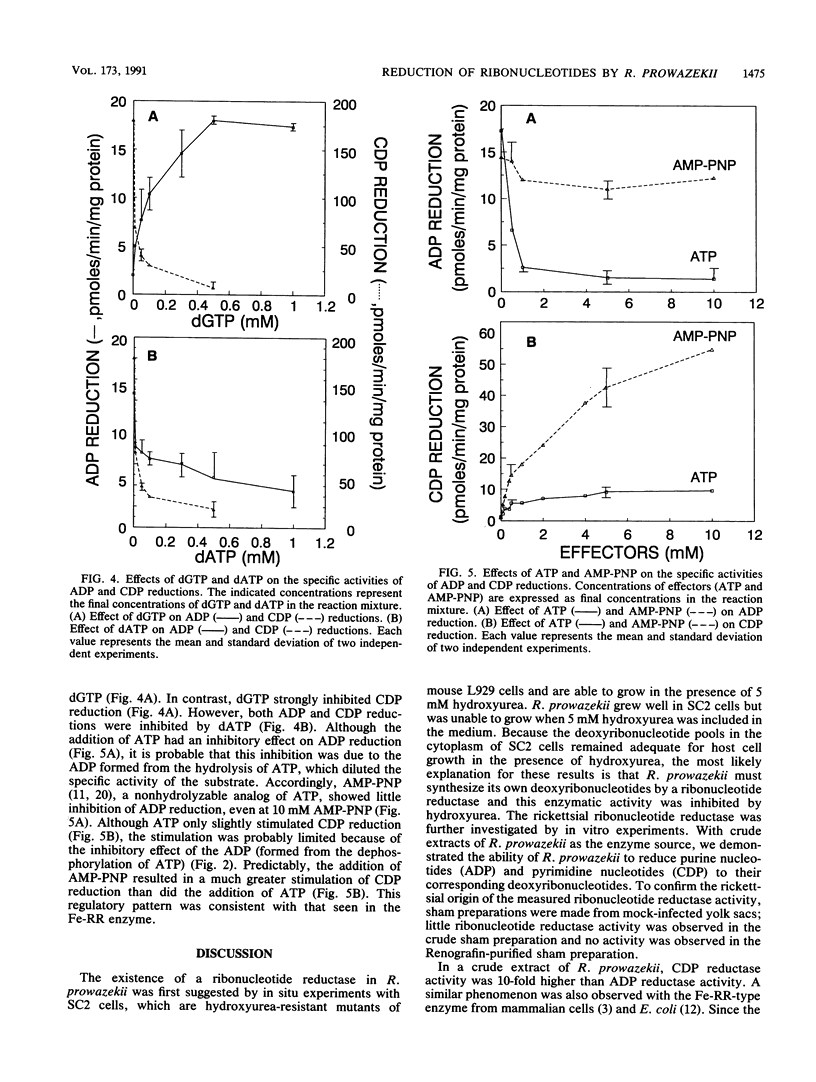
Rickettsia prowazekii, an obligate intracellular parasitic bacterium, was shown to have a ribonucleotide reductase that would allow the rickettsiae to obtain the deoxyribonucleotides needed for DNA synthesis from rickettsial ribonucleotides rather than from transport. In the presence of hydroxyurea, R. prowazekii failed to grow in mouse L929 cells or SC2 cells (a hydroxyurea-resistant cell line), which suggested that R. prowazekii contains a functional ribonucleotide reductase. This enzymatic activity was demonstrated by the conversion of ADP to dADP and CDP to dCDP, using (i) a crude extract of Renografin-purified R. prowazekii that had been harvested from infected yolk sacs and (ii) high-performance liquid chromatographic analysis. The rickettsial ribonucleotide reductase utilized ribonucleoside diphosphates as substrates, required magnesium and a reducing agent, and was inhibited by hydroxyurea. ADP reduction was stimulated by dGTP and inhibited by dATP. CDP reduction was stimulated by ATP and adenylylimido-diphosphate and inhibited by dATP and dGTP. These characteristics provided strong evidence that the rickettsial enzyme is a nonheme iron-containing enzyme similar to those found in mammalian cells and aerobic Escherichia coli.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson W. H., Winkler H. H. Transport of AMP by Rickettsia prowazekii. J Bacteriol. 1985 Jan;161(1):32–38. doi: 10.1128/jb.161.1.32-38.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow T. Evidence for a new ribonucleotide reductase in anaerobic E. coli. Biochem Biophys Res Commun. 1988 Sep 15;155(2):747–753. doi: 10.1016/s0006-291x(88)80558-8. [DOI] [PubMed] [Google Scholar]
- Cory J. G., Mansell M. M. Comparison of the cytidine 5'-diphosphate and adenosine 5'-diphosphate reductase activities of mammalian ribonucleotide reductase. Cancer Res. 1975 Sep;35(9):2327–2331. [PubMed] [Google Scholar]
- Cory J. G., Russell F. A., Mansell M. M. A convenient assay for ADP reductase activity using Dowex-1-borate columns. Anal Biochem. 1973 Oct;55(2):449–456. doi: 10.1016/0003-2697(73)90135-8. [DOI] [PubMed] [Google Scholar]
- Dasch G. A., Weiss E. Characterization of the Madrid E strain of Rickettsia prowazekii purified by renografin density gradient centrifugation. Infect Immun. 1977 Jan;15(1):280–286. doi: 10.1128/iai.15.1.280-286.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontecave M., Eliasson R., Reichard P. Oxygen-sensitive ribonucleoside triphosphate reductase is present in anaerobic Escherichia coli. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2147–2151. doi: 10.1073/pnas.86.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Hanson B. A., Wisseman C. L., Jr, Waddell A., Silverman D. J. Some characteristics of heavy and light bands of Rickettsia prowazekii on Renografin gradients. Infect Immun. 1981 Nov;34(2):596–604. doi: 10.1128/iai.34.2.596-604.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenkamp H. P. Nature and properties of the bacterial ribonucleotide reductases. Pharmacol Ther. 1983;23(3):393–405. doi: 10.1016/0163-7258(83)90020-7. [DOI] [PubMed] [Google Scholar]
- Kucera R., Paulus H. Studies on ribonucleoside-diphosphate reductase in permeable animal cells. II. Catalytic and regulatory properties of the enzyme in mouse L cells. Arch Biochem Biophys. 1982 Mar;214(1):114–123. doi: 10.1016/0003-9861(82)90013-3. [DOI] [PubMed] [Google Scholar]
- Larsson A., Reichard P. Enzymatic synthesis of deoxyribonucleotides. X. Reduction of purine ribonucleotides; allosteric behavior and substrate specificity of the enzyme system from Escherichia coli B. J Biol Chem. 1966 Jun 10;241(11):2540–2549. [PubMed] [Google Scholar]
- McClarty G. A., Chan A. K., Wright J. A. Hydroxyurea-induced conversion of mammalian ribonucleotide reductase to a form hypersensitive to bleomycin. Cancer Res. 1986 Sep;46(9):4516–4521. [PubMed] [Google Scholar]
- Moore E. C., Hurlbert R. B. The inhibition of ribonucleoside diphosphate reductase by hydroxyurea, guanazole and pyrazoloimidazole (IMPY). Pharmacol Ther. 1985;27(2):167–196. doi: 10.1016/0163-7258(85)90068-3. [DOI] [PubMed] [Google Scholar]
- Olivares J., Verdys M. Isocratic high-performance liquid chromatographic method for studying the metabolism of blood plasma pyrimidine nucleosides and bases: concentration and radioactivity measurements. J Chromatogr. 1988 Dec 29;434(1):111–121. doi: 10.1016/0378-4347(88)80066-5. [DOI] [PubMed] [Google Scholar]
- REICHARD P., BALDESTEN A., RUTBERG L. Formation of deoxycytidine phosphates from cytidine phosphates in extracts from Escherichia coli. J Biol Chem. 1961 Apr;236:1150–1157. [PubMed] [Google Scholar]
- REICHARD P. Enzymatic synthesis of deoxyribonucleotides. I. Formation of deoxycytidine diphosphate from cytidine diphosphate with enzymes from Escherichia coli. J Biol Chem. 1962 Nov;237:3513–3519. [PubMed] [Google Scholar]
- Reichard P. Interactions between deoxyribonucleotide and DNA synthesis. Annu Rev Biochem. 1988;57:349–374. doi: 10.1146/annurev.bi.57.070188.002025. [DOI] [PubMed] [Google Scholar]
- Slabaugh M. B., Johnson T. L., Mathews C. K. Vaccinia virus induces ribonucleotide reductase in primate cells. J Virol. 1984 Nov;52(2):507–514. doi: 10.1128/jvi.52.2.507-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeper J. R., Steuart C. D. A rapid assay for CDP reductase activity in mammalian cell extracts. Anal Biochem. 1970 Mar;34:123–130. doi: 10.1016/0003-2697(70)90092-8. [DOI] [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Inhibition of the growth of Rickettsia prowazekii in cultured fibroblasts by lymphokines. J Exp Med. 1983 Mar 1;157(3):974–986. doi: 10.1084/jem.157.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. Growth and physiology of rickettsiae. Bacteriol Rev. 1973 Sep;37(3):259–283. doi: 10.1128/br.37.3.259-283.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsia species (as organisms). Annu Rev Microbiol. 1990;44:131–153. doi: 10.1146/annurev.mi.44.100190.001023. [DOI] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsial permeability. An ADP-ATP transport system. J Biol Chem. 1976 Jan 25;251(2):389–396. [PubMed] [Google Scholar]


