Abstract
A prospective study was performed on 217 patients who received MIOM during corrective surgery of spinal deformities between March 2000 and December 2005. Aim is to determine the sensitivity and specificity of MIOM techniques used to monitor spinal cord and nerve root function during corrective spine surgery. MIOM is becoming an increasingly used method of monitoring function during corrective spine surgery. The combination of monitoring of ascending and descending pathways may provide more sensitive and specific results giving immediate feedback information regarding any neurological deficits during the operation. Intraoperative somatosensory spinal and cerebral evoked potentials combined with continuous EMG and motor evoked potentials of the spinal cord and muscles were evaluated and compared with postoperative clinical neurological changes. A total of 217 consecutive patients with spinal deformities of different aetiologies were monitored by means of MIOM during the surgical procedure. Out of which 201 patients presented true negative findings while one patient presented false negative and three patients presented false positive findings. Twelve patients presented true positive findings where neurological deficit after the operation was predicted. All neurological deficits in those 12 patients recovered completely. The sensitivity of MIOM applied during surgery of spinal deformities has been calculated of 92.3% and the specificity 98.5%. Based upon the results of this study MIOM is an effective method of monitoring the spinal cord and nerve root function during corrective surgery of spinal deformities and consequently improves postoperative results. The Wake-up test for surgical procedure of spinal deformities became obsolete in our institution.
Keywords: Spine surgery, Deformities, Intraoperative monitoring, Sensitivity, Specificity
Introduction
Corrective surgery in spinal deformities can lead to neurological complications, i.e. to complete or incomplete paraplegia. To reduce the risk of such complications intraoperative monitoring has been introduced. The Stagnara wake up test [12] was among the first who introduced the concept of waking the patient during the surgery to determine the functional integrity of the spinal cord. Once the patient’s neurologic condition was known, the surgeon was informed of the results and if the test was negative for paraplegia the patient was reanaesthetized and surgery was resumed. If the test was positive for paraplegia interventional strategies were initiated. The Stagnara wake up test when properly administered is 100% accurate in detecting gross motor movements, however, the patient has to be adequately awake in order to follow the commands. The major limitations of the procedure are first of all the difficulty of waking the patients and the problem associated with its repeated application and it has to be noted that the test provides information regarding the motor function only.
Tamaki [11] was among the first who used somatosensory evoked potentials for intraoperative monitoring during corrective spine surgery. Tamaki was also the one who utilised spinal evoked potentials by using epidural electrodes for stimulation and recording in order to obtain online information about the function of spinal cord, i.e. the ascending and descending pathways [11].
An extensive review of the outcome for spinal cord monitoring in deformity surgery has been presented by Nuwer [7] resulting from a survey among the members of the Scoliosis Research Society (SRS).
The large survey including 97,586 spinal surgery cases 51,263 (53%) were using SEP for monitoring purposes. Neurological deficits occurred in 0.55% and by analysing the monitoring cases the true positive findings in SEP were obtained in 0.43% and false negative findings in 0.127%.
A recent study in Germany [2] reviewed retrospectively 1,194 patients in one spine centre being operated over the period of 10 years with spinal fusion and observed that seven patients (0.59%) experienced post surgical complete or incomplete paraplegia. The same group performed an additional survey within 17 German University Clinics and designated centres for spinal surgery and surveyed 3,115 scoliosis surgeries with a frequency of iatrogenic paraplegia of 0.55%. The data was collected over a period of 10 years. The intraoperative neurological supervision in most German centres for spinal surgery (63%) during scoliosis surgery involved the wake up test immediately after repositioning. Electrophysiological monitoring by means of SEP was performed in 18% and motor evoked potentials were done only in 9% of the cases. Analysing the results which are similar to the Nuwer study the authors conclude that optimal results are found in combination of SEP and MEP examination even though wake up test is easier and more cost effective.
A question about the validity and the predictive accuracy of somatosensory evoked and motor evoked potentials were analysed by following 134 adolescent patients who underwent surgical correction of idiopathic scoliolis [6]. In this study no false negative readings were seen, however, six patients presented false positive findings. Six patients with postoperative neurologic deficits presented changes in the monitoring parameters. However, in four patients the changes returned to baseline by the end of the operation and the authors could not predict the postoperative neurologic deficit on the basis of whether monitoring changes were transient or persistent at the end of surgery. On the basis of these findings the authors believe that a wake up test should be performed for any patients with neuromonitoring changes even if the monitoring returns to baseline.
Combination of motor and sensory evoked potentials is recommended as a routine monitoring during scoliosis surgery by Luk [5] following their experience with 30 patients with no false negative case. The advantage of combination of motor and sensory evoked potentials over the SEP alone while monitoring the descending corticospinal pathways has been advocated by a Dutch spine team analysing 145 patients with motor evoked monitoring during surgery for spinal deformities [4].
The aim of the study
To analyse specificity and sensitivity for intraoperative neurophysiological monitoring (IOM) in deformity surgical procedure.
Correlate clinical outcome with the IOM findings
Patient population and method
A total of 217 patients were operated for spinal deformities with the aid of multimodal intraoperative neurophysiological monitoring (MIOM) from March 2000 till December 2005 in the spine centre of the Schulthess Clinic Zürich. The clinical diagnosis of the patients is summarized in Table 1.
Table 1.
The clinical diagnosis of 217 patients who underwent surgical procedure with the aid of multimodal intraoperative neurophysiological monitoring
| Diagnosis | Frequency | % |
|---|---|---|
| Idiopathic Scoliosis | 60 | 27.65 |
| Congenital anomalies | 25 | 11.52 |
| Degenerative scoliosis | 84 | 38.71 |
| Posttraumatic deformities | 14 | 6.45 |
| Neuromuscular deformities | 25 | 11.52 |
| Miscellaneous | 9 | 4.15 |
| Total | 217 | 100.00 |
The patients were informed about the procedure after the neurological examinations. The mean age of the patients was 40.4 years (range 2.4–84.1 years). Hundred and sixty two were female and 55 were male.
The general inclusion and exclusion criteria, and the method of examination (MIOM) as well as the anaesthesia protocol have been described by Sutter et al. [9, 10]. All MIOMs were performed by experienced neurophysiologists who are specially trained in different methods of MIOM.
Results
The surgical procedures were planned and performed according to the pre-existing pathology. The intraoperative monitoring was on average 6.3 h ranging from 1.8 to 17 h. During the surgical procedure ten different tests or modalities were applied according to the actual situation taking the surgical procedure into account in order to assess the functional status of the spinal cord, i.e. the motor and sensory pathways and if required nerve roots. The different tests and their frequency of application during the surgery are summarized in Table 2.
Table 2.
Test applied to the patient population (n = 217) with spinal deformities
| Monitoring modality | Monitorings applied | Baseline recording | ||
|---|---|---|---|---|
| Out of 217 cases | Mean tests per patient | Normal | Abnormal | |
| cm-EP | 216 (100%) | 2.8 | 120 | 96 |
| sm-EP | 78 (36%) | 2.2 | 78 (NVM) | |
| cs-EP | 87 (40%) | 1.4 | 63 | 22 |
| ss-EP | 34(16%) | 1 | 34 (NVM) | |
| nc-EP | 195 (90%) | 1.6 | 132 | 80 |
| ns-EP | 85 (39%) | 1.2 | 85 (NVM) | |
| F-wave | 2 (1%) | 2 (NVM) | ||
| Cont. EMG | 190 (88%) | 2.9 | No Spontaneous Activity | |
| Ped Screw Stim. | 56 (26%) | 3.3 | ||
Tests: recorded muscle pairs or stimulated nerve pairs in a given modality
NVM, Normative value missing; cm-EP, cerebro-muscular evoked potentials; cs-EP, cerebro-spinal evoked potentials; ns-EP, neuro-spinal evoked potentials; nc-EP, neuro-cerebral evoked potentials; sm-EP, spino-muscular evoked potentials; ss-EP, spino-spinal evoked potentials; BCR, bulbo-cavernosus reflex; BAR, bulbo-anal reflex; AEP, acoustic evoked potentials
The surgeon has been informed about the changes of the potentials particularly if the trend justified an alert so the surgeon could adapt the procedure accordingly.
Within the entire group of 217 patients undergoing corrective spinal surgery 201 patients presented true negative findings during the intraoperative monitoring while one patient presented false negative findings. This 61 year-old female with spondyloptosis L5 and secondary progressive lumbar kyphosis presented normal intraoperative monitoring during the 13 h operation. Postoperatively radiculopathy of L5 and S1 nerve root with partial sensory motor deficit on the left side has been documented. However, it could not be verified with certainty that the radiculopathy occurred during the surgical procedure or as a result of ischemia after the operation. Nevertheless according to our criteria this case clearly belongs to the false negative.
Three patients presented false positive results while pathological MIOM changes have been monitored and neurological deficits were expected postoperatively, the clinical examination, however, proved unchanged neurological status. The detailed analyses of the false negative and false positive cases are summarized in Table 3.
Table 3.
Description of cases of “false negative” findings (n = 1) and “false positive” (n = 3) out of 217 patients operated for spinal deformity
| Patient | Region | Pathology | Surgery | Duration | IOM modalities | IOM-baseline | IOM-changes | Neurological deterioration | Duration | Recovery |
|---|---|---|---|---|---|---|---|---|---|---|
| False negative cases | ||||||||||
| L.M., f., 61y | L4 − S1 | Ptosis L5/S1, secundary porgressiv lumbar kyphosis | Dorsal dekompression, correction and fusion | 12.8 h | cmP,VM,TA,AH-EP nPN,TNc-EP Ped.screw stimulation Cont. EMG |
All potentials normal | None | Radiculopathy L5 + S1 left | Persistent partial deficit | No |
| Patient | Region | Pathology | Surgery | Duration (h) | IOM modalities | IOM-baseline | IOM-changes | Expected neurological deficit | ||
|---|---|---|---|---|---|---|---|---|---|---|
| False positive cases (deformity database) | ||||||||||
| K.V., f, 12y | T4 − S1 | Scoliosis, idiopathic | Corrective surgery ventral and dorsal | 10.3 | csT11,T7-EP c/smTA,AH-EP ssEP nTNc/nTNs-EP |
Normal potentials | Loss of all signals | Metabolic disorder | ||
| R.A., f, 21y | C5 − S1 | Scoliosis, neuromuscular | Corrective surgery from dorsal | 7 | csT11-EP cmVM,TA,AH-EP smVM,TA,AH-EP nTNc-EP cont.EMG VM,TA,AH |
Normal potentials | Loss of all singnal correlated with ischaemic shock syndrome | Cortico-spinal deficits | ||
| B.E., f. 79y | L1 − L5 | Scoliosis, degenerative | Decompression and corrective spondylodesis from dorsal | 7 | cmP,VM,PL-EP smP,VM,PL-EP EMGP,VM,PL nPN,SaphNc-EP |
All motor and sensory potential pathological available | Threshold stimulation identified screws L2&3 left with recessus perforation | L2 and L3 radiculopathy left | ||
During the intraoperative monitoring of 12 cases (5.5%) true positive cases presented intraoperatively pathological changes of the monitoring parameters and new neurological deficits after the operation have been confirmed. The detailed analysis of the cases is documented in Table 4. These 12 cases represent a patient population with severe scoliosis of degenerative, posttraumatic or neuromuscular origin. One scoliosis case was a result of congenital anomaly and one severe scoliosis case due to neuromuscular changes and arthrogryposis multiplex. The duration of the operation and the surgical procedures applied indirectly document the severity of the preoperative condition. In all 12 cases at a certain moment of the operation pathological changes of the monitoring parameters and or deterioration of pre-existing pathological parameters occurred and were immediately communicated to the spine surgeon in order to adapt the surgical procedure accordingly. During the surgical procedure of those cases particularly after occurrence of pathological changes an intense communication with discussion of potential origin of the neurophysiological changes took place between the neurophysiologist and the spine surgeon. The surgical procedure was completed despite the suspected neurological deterioration, however, in ten of the 12 cases no indication has been observed to justify the suspicion of complete lesion of the ascending and/or descending pathways. Out of the 12 cases eight patients recovered within hours, three additional patients within 3 months. Even though five patients presented postoperatively partial or mild paraparesis of the lower extremities all of them recovered within hours (1 patient within 6 weeks). Out of the 217 patients including 12 patients presenting true positive findings in IOM none of them sustained a permanent neurological deficit as a result of corrective surgery due to the spinal deformity.
Table 4.
Description of cases of “true positive” findings (n = 12) out of 217 patients operated for spinal deformity
| Region | Pathology | Surgery | Duration hours | IOM modality | Baseline exam | Changes of MIOM | Neurological deterioration | Duration | Recovery | |
|---|---|---|---|---|---|---|---|---|---|---|
| True positive cases | ||||||||||
| R.A., f, 54y | T8 − L3 | Scoliosis postraumatic | Decompression, correction and fusion, dvd | 12.6 | csT10EP cmTA,AHEP sT10mTA,AHEP nPNsT10EP |
All available, but pathologic | After distraction T12 − L1 loss of all potentials of cmEP | Deterioration of existing partialConus syndrome | ? | ? |
| H.S., f, 75y | L3 − S1 | Scoliosis degenerative | Decompression and fusion, d | 3.0 | cmVM,TA,AHEP nPNcEP cont EMGVM,TA,AH |
cmVM,TAEP right not available | Reduction of amplitude of cmEP VM right during decompression | Partial sensomotor deficits L4 right | Hours | Complete |
| J.K., f, 44y | T5 − L2 | Scoliosis after tumour resection | Columnotomy correction and fusion, d | 7.25 | csT7,L1EP cmVM,TAEP sL1mVM,TAEP sL1sT7EP nTNsT7EP nTNcEP cont EMGVM,TA |
All available | At the end of columnotomy loss of cmEP, reduction of D-wave. Continuous recovery of D-wave, but pathologic cmEP at the end of operation | Partial paraparesis | Hours | Complete |
| S.K., f, 74y | T7 − L3 | Scoliosis neuromuscular | Decompression, osteotomie T12, correction and fusion, d | 9.5 | csL1EP cmVM,TAEP nFN,PNsL1EP nFN,PNcEP cont. EMGVM,TA |
All available, except not reproducible ncEP | Alteration of cmEP of VM left during ped. screwing | Mild partial sensomotor deficits L3 left | Hours | Complete |
| F.C., m, 57y | T9 − L2 | Scoliosis posttraumatic | Correction and fusion, dvd | 10.0 | csT8,L1EP cmVM,TA,AH,BREP smVM,TA,AH,BREP sT8sL1EP nTN,MNsL1,T8EP nTN,MNcEP cont. EMGVM,TA,AH,BR |
Normal cmEP, pathologic ncEP | Fading away of all cmEP | Cortical event (disturbance of conciousness) | Hours | Complete |
| R.G., w, 80y | T5 − L5 | Scoliosis degenerative | Decompression, correction and fusion | 5.5 | cmVM,otherEP nFNcEP cont. EMGVM,other |
All available, but pathologic | After correction massive reduction of amplitude of cmEP VM, partial recovery | Partial sensomotor deficits L3 + L4 | Hours | Complete |
| N.G., m, 8y | L4 − S2 | Scoliosis congenital anomaly | Correction and fusion, d | 8.0 | cmVM,TA,AHEP nTNcEP cont. EMGVM,TA,AH Ped. screw stimulation |
Pathological baseline for all modalities | Reduction of amplitude of cmEP during correction | Partial paraparesis | 6 weeks | Complete |
| W.C., f, 14y | T3 − L2 | Scoliosis idiopathic | Correction and fusion, d | 3.75 | csT1,T12EP cmTA,AHEP sT1mTA,AHEP nTNcEP nTNsT12EP cont. EMGTA,AH |
All available | Associated with bleeding partial loss of motor and sensory EP, no recovery | Partial paraparesis | 2 h | Complete |
| Y.F., m, 16y | T2 − L2 | Scoliosis congenital anomaly | Correction and fusion, d | 5.0 | csT10,C5EP cmTA,AHEP smTA,AHEP sT0sC5EP nTNsC5EP nTNcEP cont. EMGTA,AH |
All available, but pathologic | Loss of all EP associated to massive blood loss, only partial recovery of EP | Partial paraparesis | Hours | Complete |
| P.L., f, 13y | T3 − L2 | Scoliosis | Correction and fusion, d | 4.25 | csT12,T8EP cmTA,AHEP sT12mTA,AHEP nTNsT12,8EP nTNcEP cont. EMGTA,AH |
All available | Partial loss of all EP, no recovery. | Partial paraparesis | Hours | Complete |
| M.R., m, 16y | L3 − L5 | Scoliosis neuromusculararthrogryposis | Correction and fusion, d | 2.6 | cmVM,TAEP nPNcEP cont. EMGVM, TA |
Normal cmEP, pathologic ncEP | Loss of all EP. | Cardiopulmonary decompensation due to intravasal coagulopathy | Exitus letalis | |
| V.L., m, 73y | C2 − T8 | Scoliosis neuromuscular | Osteotomie C7/T1, correction and fusion, d | 7.0 | cmTA,D,TM,ADMEP nMN,TNcEP cont. EMGTA,D,TM,ADM |
All available, but pathologic | Massive alteration of cmEP TM right associated to correction, no recovery | Partial sensomotor deficit C7 right | 3 months | Complete |
The onset of new neurological deficit could be predicted based upon IOM findings
Unfortunately one of the patients in the deformity group died during the operation and after unsuccessful cardiopulmonary resuscitation. This patient suffered a severe neuromuscular scoliosis as a result of arthrogryposis multiplex. The operation has been planned as correction of the scoliosis with dorsal fusion. Approximately 1 h and 30 min from the onset of the operation uncontrolled general bleeding occurred due to intravasal disseminating coagulopathy.
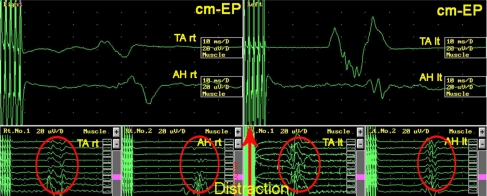
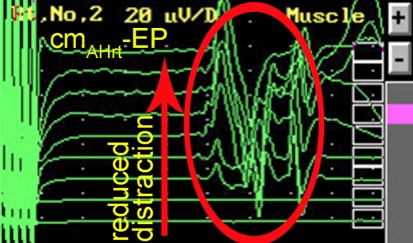
To document the interaction between the spine surgeon and neurophysiologist resulting in a successful management of potential complications due to the corrective surgery is documented in a case of a 54 year-old female with posttraumatic scoliosis of thoracolumbar junction with preexisting partial conus syndrome (Fig. 1) which was the actual indication for decompression and correction of the deformity. The duration of the operation was 12.5 h. After dorsal decompression without any functional problems the patient developed after ventral corpectomy and correction of the kyphosis progressive alteration of the motor-evoked potentials (Fig. 2) probably due to distraction of the nerve roots. After release within the operated segment the motor-evoked potentials improved again to base-line parameters (Fig. 3). The patient had normal clinical motor function, however, a slight deterioration of preexisting partial conus syndrome which recovered to the preexisting status within 3 months (Fig. 4).
Fig. 1.
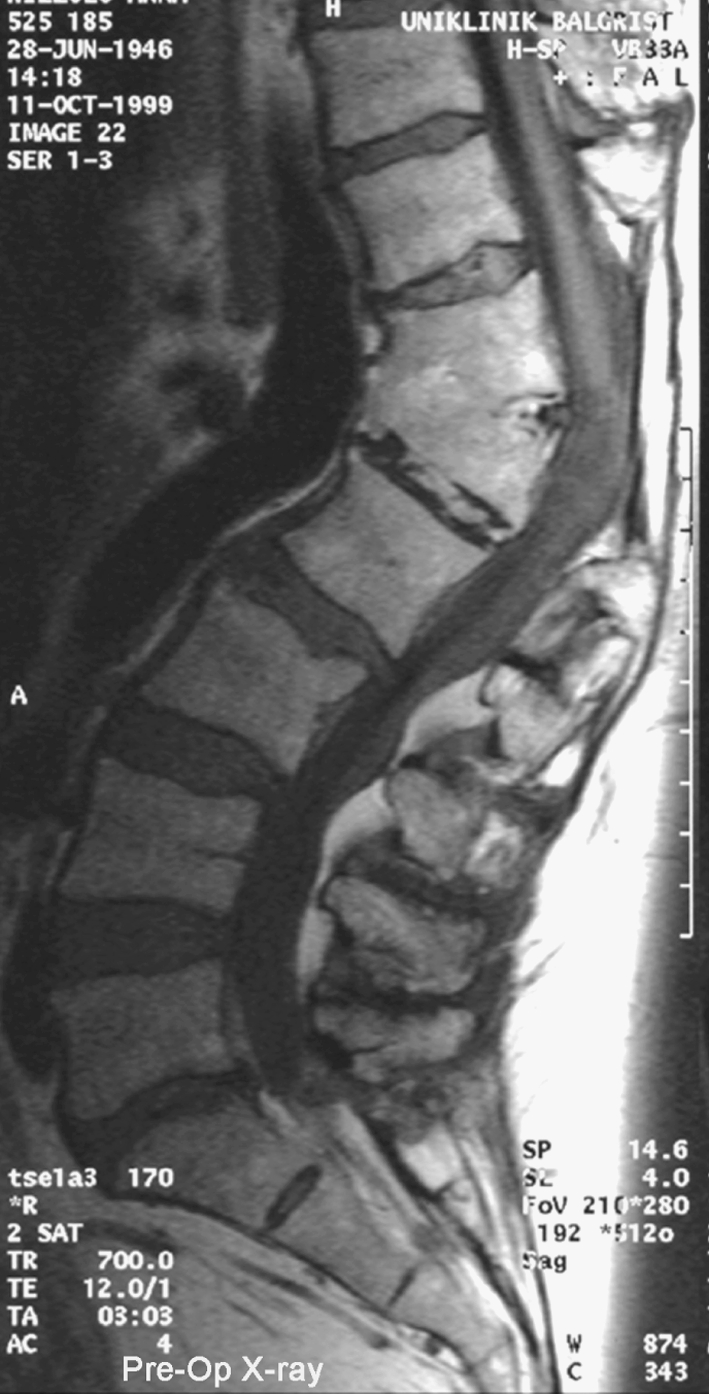
Pre-OP X-ray
Fig. 2.
Alteration of cm-EP tibial anterior and abductor hallucis muscles due to segmental distraction and correction of deformity
Fig. 3.
Recovery of cm-EP after reduction of segmental distraction
Fig. 4.
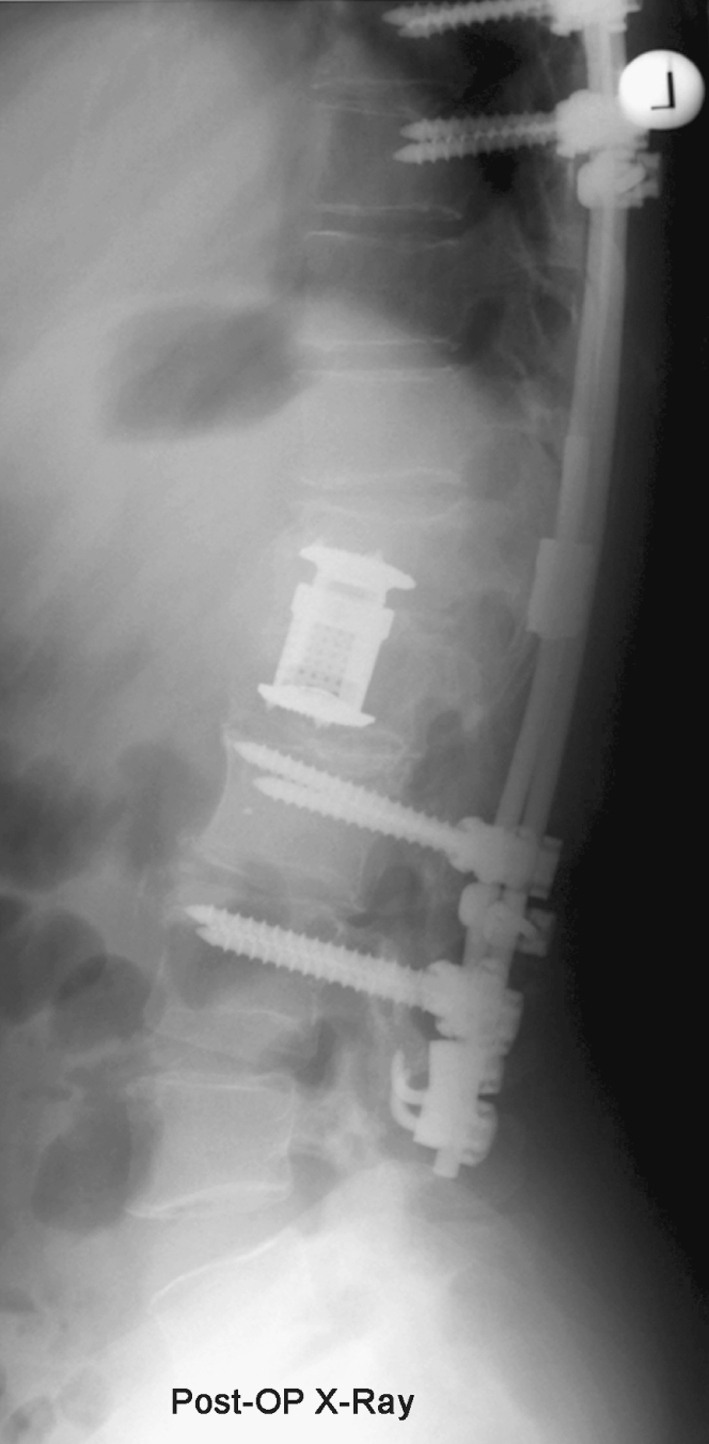
X-ray after correction of deformity
Applying the standard formula sensitivity of the intraoperative monitoring during surgery of spinal deformities has been calculated of 92.3% (95% confidence interval: 62–99.6%) and specificity of 98.5% (95% confidence interval: 95–99.6%).
Discussion
The surgical correction of spinal deformities has a long history and in most of the cases particularly in the progressive idiopathic scoliosis the criteria for indication for surgical correction are well established. The advancement of the surgical techniques in the correction of deformities of different origin and ethiology often combined with decompressive procedures created the demand for monitoring the function of spinal cord and/or nerve roots if potentially at risk during the surgical procedure. The Stagnara Wake-up test [12] was about the first who introduced the concept of monitoring the surgical procedure by waking the patient during the surgery to determine the functional integrity of the spinal cord. However, the limitation of such procedure is well known and particularly such a test provides information regarding the motor function only. Nevertheless the recent study in major German spine centres [2] document that 63% of patients undergoing surgery for correction of scoliosis are still using the Stagnara Wake-up test as a means of monitoring of the surgical procedure. The electrophysiological monitoring by means of SEP was performed in 18% and the motor evoked potentials by transcranial cortex stimulation were done in Germany only in 9% of the cases (study published in 2005). In the 10-year period this study revealed that 0.59% of operated patients experienced postsurgical complete or incomplete paraplegia. A similar result previously published as a result of an extensive review of the outcome for spinal cord monitoring in deformity surgery among the members of the Scoliosis Research Society [7]. At that time only sensory evoked potentials were available for monitoring. From the entire group 0.55% presented postoperative neurological deficits with 0.127% of false negative findings and 0.43% of true positive findings.
The later developments of the neurophysiological methods clearly indicated that combined application of motor and sensory evoked potentials will increase the validity and reliability of the monitoring techniques and has been advocated by several studies [4–6].
The major advancement of monitoring techniques being dormant for a long time, was introduced by Tamaki with application of epidural electrodes which allow to monitor D-wave [1] as a more or less online monitoring of the function of descending corticospinal pathways, the major concern of the spine surgeon. The concept of monitoring guiding surgery in spinal disorders, particularly in intramedullary tumour was introduced by the group of Epstein in the 1980s and 1990s [8, 3] which was introduced in our institution in the late 1990s by personal communication from both pioneering groups (Tamaki, Epstein). The close collaboration between neurologist specially trained in the monitoring techniques and the spine surgeon already during the preoperative planning stage facilitated the communication during the surgical procedure. The neurologist responsible for the monitoring decides from the multimodal approach which neural structure has to be stimulated in order to monitor the appropriate evoked potentials to obtain the functional status of the spinal cord in the descending or ascending pathways and if required the function of the motor or sensory part of the nerve roots. According to the trend analysis of the development of the different monitoring modalities in the course of the surgical procedure, particularly at the critical moments such as correction of the deformity allows the surgeon to go to the limit in order to obtain the best possible correction without taking the risk of damaging the neural structures. During the entire period being documented in this prospective study, not a single Wake-up test was necessary to obtain an information about the function of the spinal cord. The one false negative case occurred during an operation of severe spondyloptosis with postoperative radiculopathy on one side of L5 and S1 root and the three false positive cases document the validity and reliability of the multimodal intraoperative monitoring in corrective surgery of spinal deformities with sensitivity of 92.3% and specificity of 98.5% which exceeds the previously published studies where one or two modalities were applied. The detailed analysis of the true positive cases, in our series 12 patients, documents the close collaboration between the neurologist and spine surgeons as the neurologist is responsible for the monitoring and communication of the function of the spinal cord and alerts or warns the surgeon when neurostructures are at risk so that the surgeon can adapt his or her procedure accordingly.
The results of the presented study document the advantage of the application of multimodal intraoperative monitoring during surgery of spinal deformities if appropriately performed by a specially trained neurologist or neurophysiologist with not a single case of surgery-related persisting neurological deficits. We can confirm the previous studies by Luk [5] and Langeloo [4] recommend the application of multimodal intraoperative monitoring during this kind of surgical procedures, which make the Stagnara Wake-up test with the currently available technology and practical experience obsolete. The one unfortunate case suffered from arthrogryposis multiplex with lethal complication is not direct result of the corrective surgery but unforeseen vascular complication with disseminating intravasal bleeding.
Acknowledgments
Dr. Lote Medicus fund for the financial support of the development of MIOM at the Schulthess Clinic. Dave O’Riordan and Charles McCammon for helping with the manuscript. Anne Mannion PhD for the critical review of the manuscript.
Conflict of interest statement None of the authors has any potential conflict of interest.
References
- 1.Amassian VE, Stewart M, Quirk GJ, Rosenthal JL. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 1987;20:74–93. [PubMed] [Google Scholar]
- 2.Delank KS, Delank HW, Konig DP, Popken F, Furderer S, Eysel P. Iatrogenic paraplegia in spinal surgery. Arch Orthop Trauma Surg. 2005;125:33–41. doi: 10.1007/s00402-004-0763-5. [DOI] [PubMed] [Google Scholar]
- 3.Kothbauer K, Deletis V, Epstein F (1998) Motor evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. http://www.aans.org/journals/online_j/may98/4-5-1 [DOI] [PubMed]
- 4.Langeloo DD, Lelivelt A, Louis Journee H, Slappendel R, Kleuver M. Transcranial electrical motor-evoked potential monitoring during surgery for spinal deformity: a study of 145 patients. Spine. 2003;28:1043–1050. doi: 10.1097/00007632-200305150-00017. [DOI] [PubMed] [Google Scholar]
- 5.Luk KD, Hu Y, Wong YW, Cheung KM. Evaluation of various evoked potential techniques for spinal cord monitoring during scoliosis surgery. Spine. 2001;26:1772–1777. doi: 10.1097/00007632-200108150-00008. [DOI] [PubMed] [Google Scholar]
- 6.Noonan KJ, Walker T, Feinberg JR, Nagel M, Didelot W, Lindseth R. Factors related to false- versus true-positive neuromonitoring changes in adolescent idiopathic scoliosis surgery. Spine. 2002;27:825–830. doi: 10.1097/00007632-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Nuwer MR, Dawson EG, Carlson LG, Kanim LE, Sherman JE. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96:6–11. doi: 10.1016/0013-4694(94)00235-D. [DOI] [PubMed] [Google Scholar]
- 8.Sala F, Krzan MJ, Deletis V. Intraoperative neurophysiological monitoring in pediatric neurosurgery: why, when, how? Childs Nerv Syst. 2002;18:264–287. doi: 10.1007/s00381-002-0582-3. [DOI] [PubMed] [Google Scholar]
- 9.Sutter M, Eggspühler A, Grob D, Jeszenszky D, Porchet F, Muller A, Dvorak J (2007) The diagnostic value of multimodal intraoperative monitoring (MIOM) during spine surgery: a prospective Study of 1017 cases. Eur Spine J (in press) [DOI] [PMC free article] [PubMed]
- 10.Sutter M, Eggspühler A, Muller A, Dvorak J (2007) Multimodal intraoperative monitoring: methodology. Eur Spine J (in preperation) [DOI] [PMC free article] [PubMed]
- 11.Tamaki T, Noguchi T, Takano H, Tsuji H, Nakagawa T, Imai K, Inoue S. Spinal cord monitoring as a clinical utilization of the spinal evoked potential. Clin Orthop Relat Res. 1984;184:58–64. [PubMed] [Google Scholar]
- 12.Vauzelle C, Stagnara P, Jouvinroux P. Functional monitoring of spinal cord activity during spinal surgery. Clin Orthop Relat Res. 1973;93:173–178. doi: 10.1097/00003086-197306000-00017. [DOI] [PubMed] [Google Scholar]