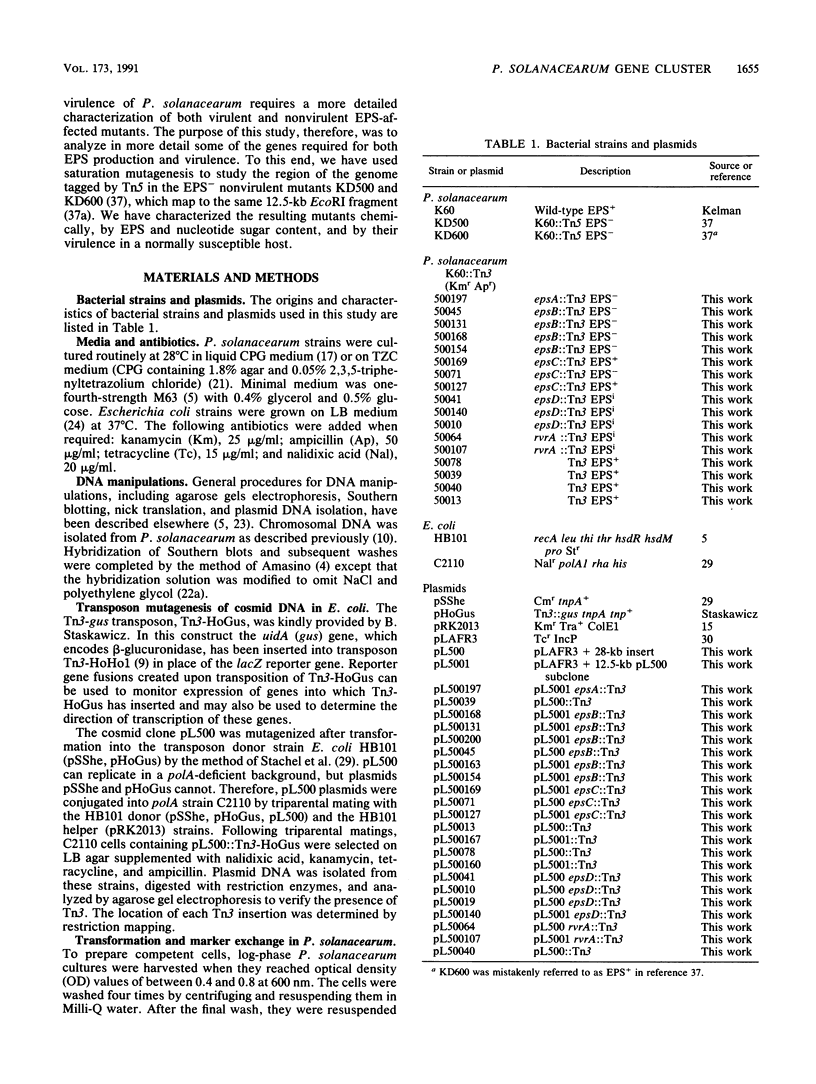
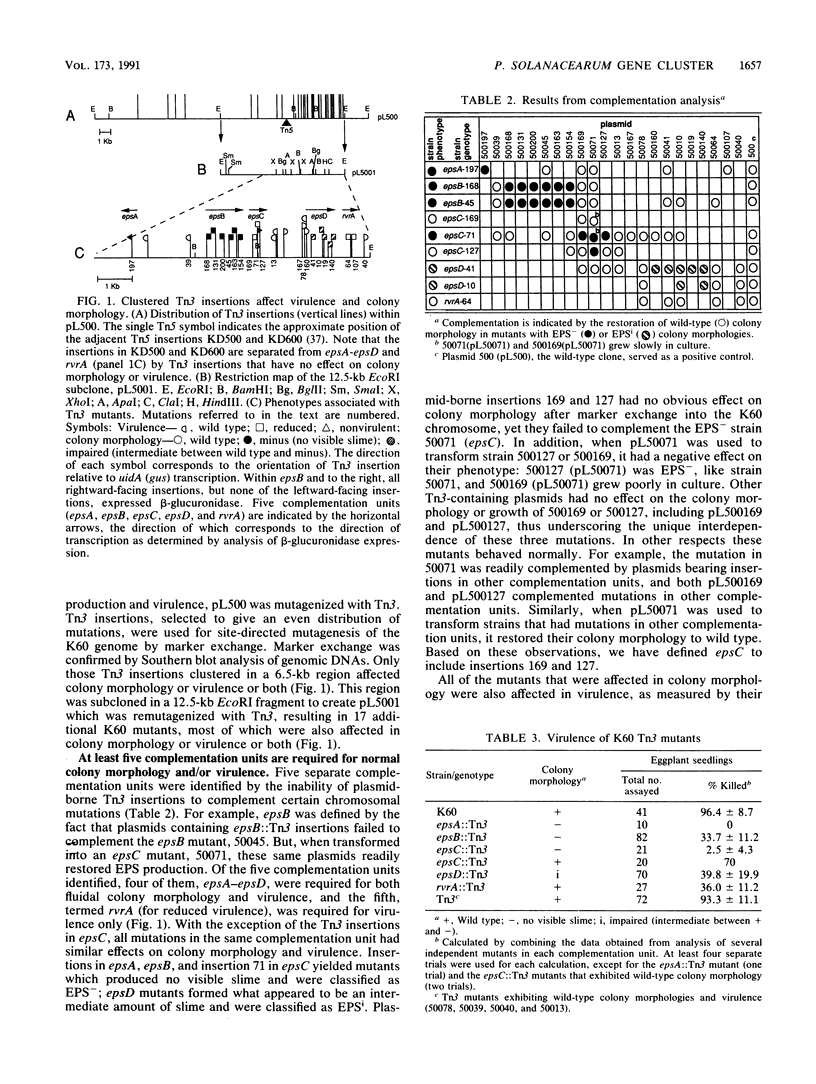
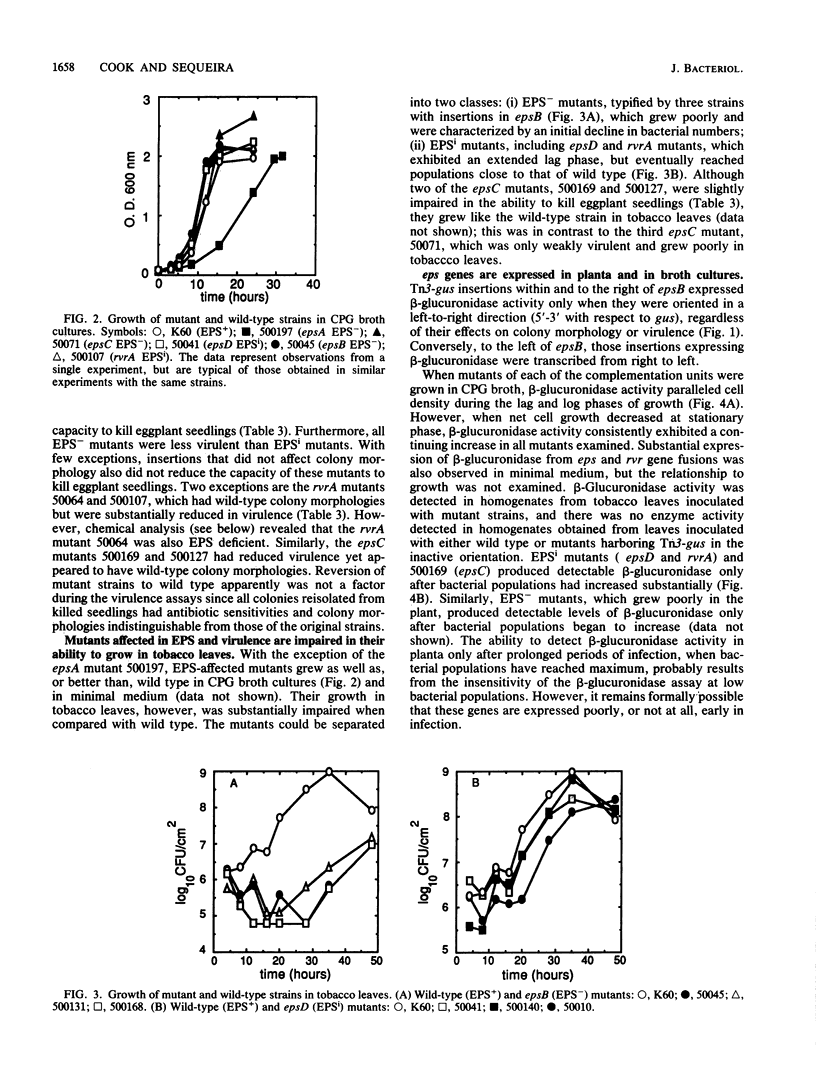
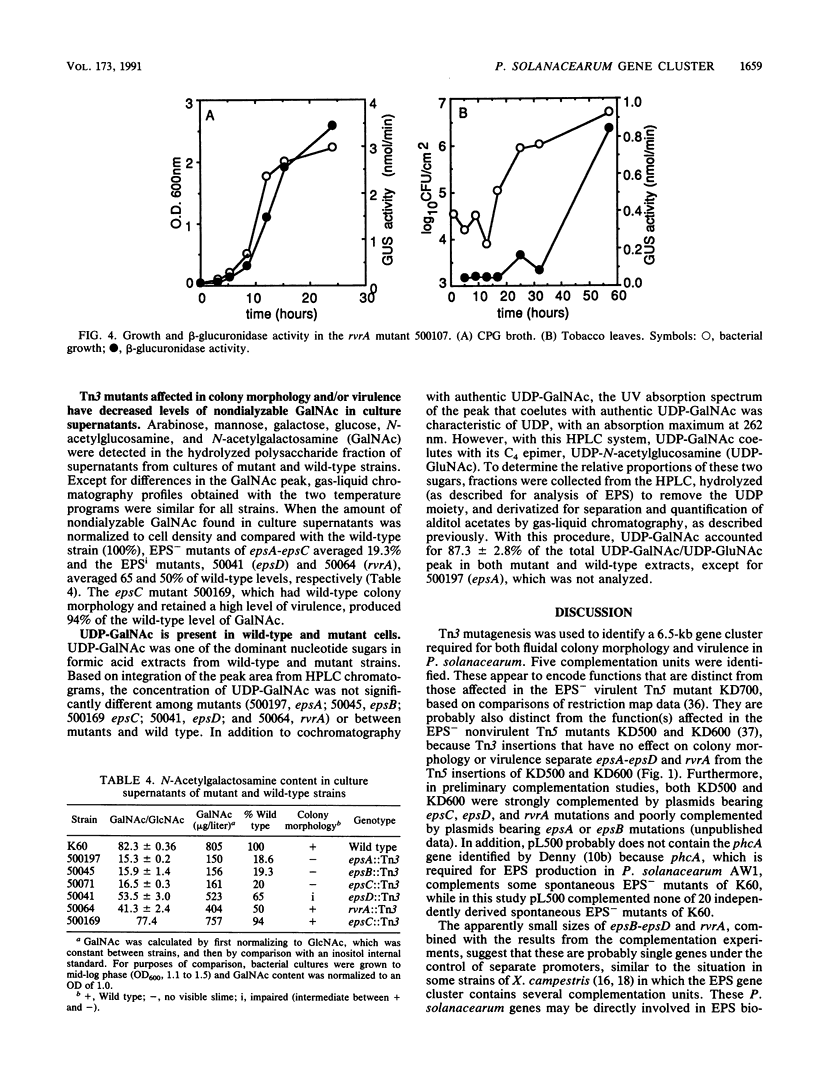
Abstract
Infection of host plants by Pseudomonas solanacerum results in wilting, which is thought to be due largely to the occlusion of xylem vessels by the P. solanacearum extracellular polysaccharide (EPS) that primarily consists of N-acetylgalactosamine (GalNAc). By means of Tn3 mutagenesis, we identified a 6.5-kb gene cluster that contains five complementation units required for EPS production and virulence in this bacterium. There was positive correlation between the amount of EPS produced in culture and (i) in planta growth and (ii) virulence. Based on analysis of beta-glucuronidase-gene fusions, these genes are expressed both in broth cultures and in planta and may be constitutive. Both wild-type and mutant strains contained similar amounts of UDP-GalNAc, the predicted primary substrate for EPS synthesis. Thus, the EPS mutants we obtained should be useful in the analysis of steps in the assembly of the polysaccharide and how this process is related to virulence.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amasino R. M. Acceleration of nucleic acid hybridization rate by polyethylene glycol. Anal Biochem. 1986 Feb 1;152(2):304–307. doi: 10.1016/0003-2697(86)90413-6. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Neilson M. J., Sequeira L., Keegstra K. G. Chemical Characterization of the Lipopolysaccharide of Pseudomonas solanacearum. Appl Environ Microbiol. 1984 May;47(5):1096–1100. doi: 10.1128/aem.47.5.1096-1100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Ames B. N. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem. 1982 Aug 25;257(16):9759–9769. [PubMed] [Google Scholar]
- Dolph P. J., Majerczak D. R., Coplin D. L. Characterization of a gene cluster for exopolysaccharide biosynthesis and virulence in Erwinia stewartii. J Bacteriol. 1988 Feb;170(2):865–871. doi: 10.1128/jb.170.2.865-871.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drigues P., Demery-Lafforgue D., Trigalet A., Dupin P., Samain D., Asselineau J. Comparative studies of lipopolysaccharide and exopolysaccharide from a virulent strain of Pseudomonas solanacearum and from three avirulent mutants. J Bacteriol. 1985 May;162(2):504–509. doi: 10.1128/jb.162.2.504-509.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easson D. D., Jr, Sinskey A. J., Peoples O. P. Isolation of Zoogloea ramigera I-16-M exopolysaccharide biosynthetic genes and evidence for instability within this region. J Bacteriol. 1987 Oct;169(10):4518–4524. doi: 10.1128/jb.169.10.4518-4524.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding N. E., Cleary J. M., Cabañas D. K., Rosen I. G., Kang K. S. Genetic and physical analyses of a cluster of genes essential for xanthan gum biosynthesis in Xanthomonas campestris. J Bacteriol. 1987 Jun;169(6):2854–2861. doi: 10.1128/jb.169.6.2854-2861.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick C. A., Sequeira L. Lipopolysaccharide-Defective Mutants of the Wilt Pathogen Pseudomonas solanacearum. Appl Environ Microbiol. 1984 Jul;48(1):94–101. doi: 10.1128/aem.48.1.94-101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hötte B., Rath-Arnold I., Pühler A., Simon R. Cloning and analysis of a 35.3-kilobase DNA region involved in exopolysaccharide production by Xanthomonas campestris pv. campestris. J Bacteriol. 1990 May;172(5):2804–2807. doi: 10.1128/jb.172.5.2804-2807.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelman A., Hruschka J. The role of motility and aerotaxis in the selective increase of avirulent bacteria in still broth cultures of Pseudomonas solanacearum. J Gen Microbiol. 1973 May;76(1):177–188. doi: 10.1099/00221287-76-1-177. [DOI] [PubMed] [Google Scholar]
- Roberts D. P., Denny T. P., Schell M. A. Cloning of the egl gene of Pseudomonas solanacearum and analysis of its role in phytopathogenicity. J Bacteriol. 1988 Apr;170(4):1445–1451. doi: 10.1128/jb.170.4.1445-1451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., An G., Flores C., Nester E. W. A Tn3 lacZ transposon for the random generation of beta-galactosidase gene fusions: application to the analysis of gene expression in Agrobacterium. EMBO J. 1985 Apr;4(4):891–898. doi: 10.1002/j.1460-2075.1985.tb03715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskawicz B., Dahlbeck D., Keen N., Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987 Dec;169(12):5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmasky M. E., Staneloni R. J., Leloir L. F. Lipid-bound saccharides in Rhizobium meliloti. J Biol Chem. 1982 Jun 25;257(12):6751–6757. [PubMed] [Google Scholar]
- Van Alfen N. K., McMillan B. D., Turner V., Hess W. M. Role of pit membranes in macromolecule-induced wilt of plants. Plant Physiol. 1983 Dec;73(4):1020–1023. doi: 10.1104/pp.73.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteheart S. W., Passaniti A., Reichner J. S., Holt G. D., Haltiwanger R. S., Hart G. W. Glycosyltransferase probes. Methods Enzymol. 1989;179:82–95. doi: 10.1016/0076-6879(89)79116-3. [DOI] [PubMed] [Google Scholar]
- Xu P. L., Iwata M., Leong S., Sequeira L. Highly virulent strains of Pseudomonas solanacearum that are defective in extracellular-polysaccharide production. J Bacteriol. 1990 Jul;172(7):3946–3951. doi: 10.1128/jb.172.7.3946-3951.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P. L., Leong S., Sequeira L. Molecular cloning of genes that specify virulence in Pseudomonas solanacearum. J Bacteriol. 1988 Feb;170(2):617–622. doi: 10.1128/jb.170.2.617-622.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


