Abstract
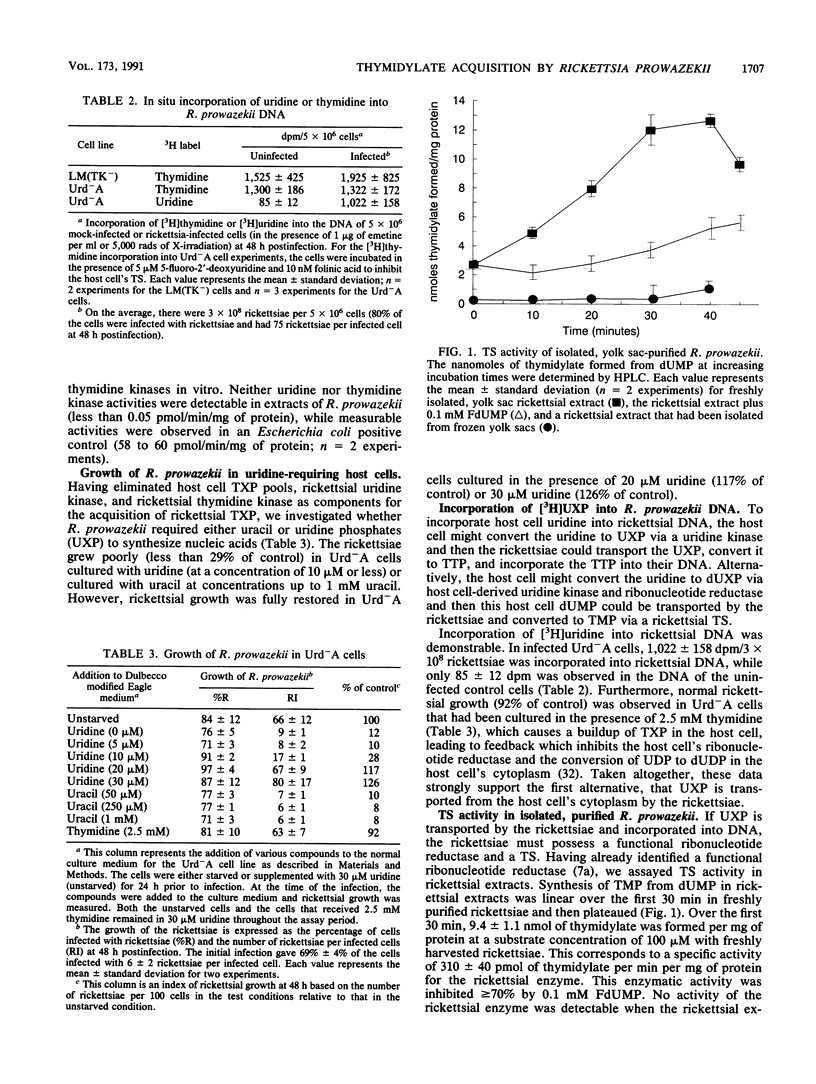
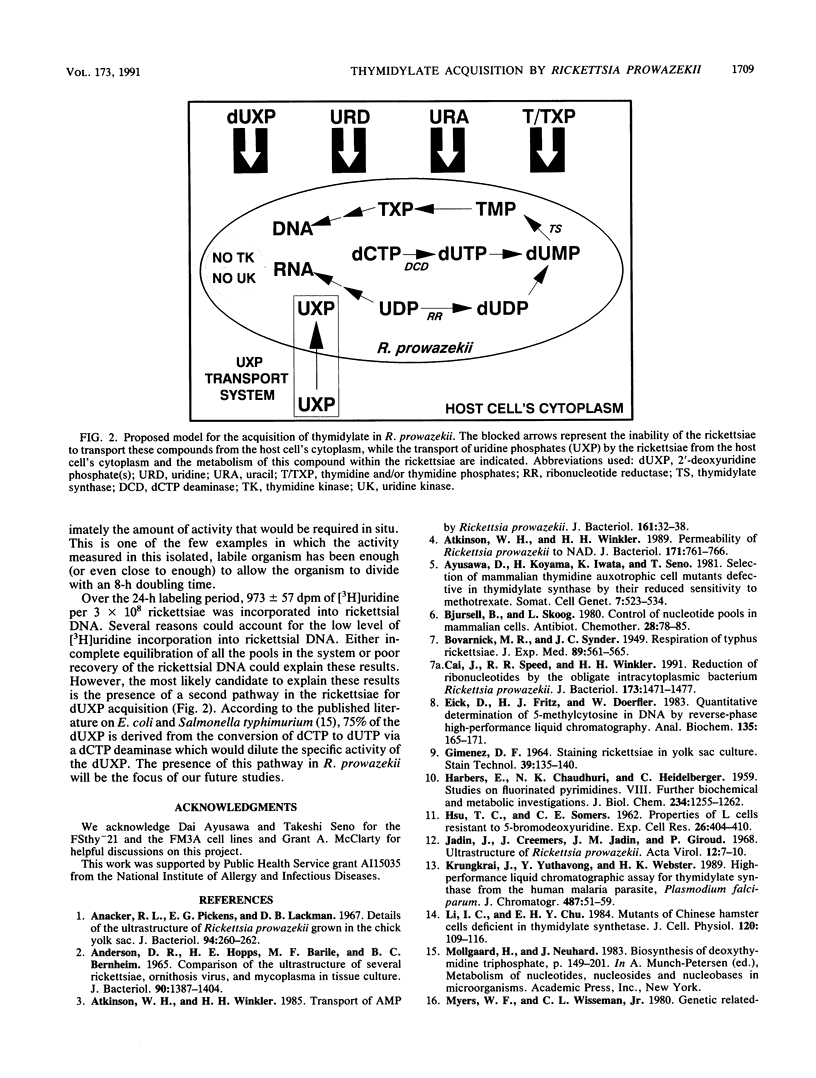
The pathway for the acquisition of thymidylate in the obligate bacterial parasite Rickettsia prowazekii was determined. R. prowazekii growing in host cells with or without thymidine kinase failed to incorporate into its DNA the [3H]thymidine added to the culture. In the thymidine kinase-negative host cells, the label available to the rickettsiae in the host cell cytoplasm would have been thymidine, and in the thymidine kinase-positive host cells, it would have been both thymidine and TMP. Further support for the inability to utilize thymidine was the lack of thymidine kinase activity in extracts of R. prowazekii. However, [3H]uridine incorporation into the DNA of R. prowazekii was demonstrable (973 +/- 57 dpm/3 x 10(8) rickettsiae). This labeling of rickettsial DNA suggests the transport of uracil, uridine, uridine phosphates (UXP), or 2'-deoxyuridine phosphates, the conversion of the labeled precursor to thymidylate, and subsequent incorporation into DNA. This is supported by the demonstration of thymidylate synthase activity in extracts of R. prowazekii. The enzyme was determined to have a specific activity of 310 +/- 40 pmol/min/mg of protein and was inhibited greater than or equal to 70% by 5-fluoro-dUMP. The inability of R. prowazekii to utilize uracil was suggested by undetectable uracil phosphoribosyltransferase activity and by its inability to grow (less than 10% of control) in a uridine-starved mutant cell line (Urd-A) supplemented with 50 microM to 1 mM uracil. In contrast, the rickettsiae were able to grow in Urd-A cells that were uridine starved and supplemented with 20 microM uridine (117% of control). However, no measurable uridine kinase activity could be measured in extracts of R. prowazekii. Normal rickettsial growth (92% of control) was observed when the host cell was blocked with thymidine so that the host cell's dUXP pool was depressed to a level inadequate for growth and DNA synthesis in the host cell. Taken together, these data strongly suggest that rickettsiae transport UXP from the host cell's cytoplasm and that they synthesize TTP from UXP.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., Pickens E. G., Lackman D. B. Details of the ultrastructure of Rickettsia prowazekii grown in the chick yolk sac. J Bacteriol. 1967 Jul;94(1):260–262. doi: 10.1128/jb.94.1.260-262.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. R., Hopps H. E., Barile M. F., Bernheim B. C. Comparison of the ultrastructure of several rickettsiae, ornithosis virus, and Mycoplasma in tissue culture. J Bacteriol. 1965 Nov;90(5):1387–1404. doi: 10.1128/jb.90.5.1387-1404.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson W. H., Winkler H. H. Permeability of Rickettsia prowazekii to NAD. J Bacteriol. 1989 Feb;171(2):761–766. doi: 10.1128/jb.171.2.761-766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson W. H., Winkler H. H. Transport of AMP by Rickettsia prowazekii. J Bacteriol. 1985 Jan;161(1):32–38. doi: 10.1128/jb.161.1.32-38.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayusawa D., Koyama H., Iwata K., Seno T. Selection of mammalian thymidine auxotrophic cell mutants defective in thymidylate synthase by their reduced sensitivity to methotrexate. Somatic Cell Genet. 1981 Sep;7(5):523–534. doi: 10.1007/BF01549656. [DOI] [PubMed] [Google Scholar]
- Bjursell G., Skoog L. Control of nucleotide pools in mammalian cells. Antibiot Chemother (1971) 1980;28:78–85. doi: 10.1159/000386063. [DOI] [PubMed] [Google Scholar]
- Cai J., Speed R. R., Winkler H. H. Reduction of ribonucleotides by the obligate intracytoplasmic bacterium Rickettsia prowazekii. J Bacteriol. 1991 Feb;173(4):1471–1477. doi: 10.1128/jb.173.4.1471-1477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick D., Fritz H. J., Doerfler W. Quantitative determination of 5-methylcytosine in DNA by reverse-phase high-performance liquid chromatography. Anal Biochem. 1983 Nov;135(1):165–171. doi: 10.1016/0003-2697(83)90746-7. [DOI] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- HARBERS E., CHAUDHURI N. K., HEIDELBERGER C. Studies on fluorinated pyrimidines. VIII. Further biochemical and metabolic investigations. J Biol Chem. 1959 May;234(5):1255–1262. [PubMed] [Google Scholar]
- HSU T. C., SOMERS C. E. Properties of L cells resistant to 5-bromodeoxyuridine. Exp Cell Res. 1962 Mar;26:404–410. doi: 10.1016/0014-4827(62)90192-1. [DOI] [PubMed] [Google Scholar]
- Jadin J., Creemers J., Jadin J. M., Giroud P. Ultrastructure of Rickettsia prowazeki. Acta Virol. 1968 Jan;12(1):7–10. [PubMed] [Google Scholar]
- Krungkrai J., Yuthavong Y., Webster H. K. High-performance liquid chromatographic assay for thymidylate synthase from the human malaria parasite, Plasmodium falciparum. J Chromatogr. 1989 Jan 27;487(1):51–59. doi: 10.1016/s0378-4347(00)83006-6. [DOI] [PubMed] [Google Scholar]
- Li I. C., Chu E. H. Mutants of Chinese hamster cells deficient in thymidylate synthetase. J Cell Physiol. 1984 Aug;120(2):109–116. doi: 10.1002/jcp.1041200202. [DOI] [PubMed] [Google Scholar]
- Patterson D., Carnright D. V. Biochemical genetic analysis of pyrimidine biosynthesis in mammalian cells: I. Isolation of a mutant defective in the early steps of de novo pyrimidine synthesis. Somatic Cell Genet. 1977 Sep;3(5):483–495. doi: 10.1007/BF01539120. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R. Toxoplasma gondii: the enzymic defect of a mutant resistant to 5-fluorodeoxyuridine. Exp Parasitol. 1978 Feb;44(1):26–35. doi: 10.1016/0014-4894(78)90077-2. [DOI] [PubMed] [Google Scholar]
- Smith D. K., Winkler H. H. Characterization of a lysine-specific active transport system in Rickettsia prowazeki. J Bacteriol. 1977 Mar;129(3):1349–1355. doi: 10.1128/jb.129.3.1349-1355.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed R. R., Winkler H. H. Acquisition of polyamines by the obligate intracytoplasmic bacterium Rickettsia prowazekii. J Bacteriol. 1990 Oct;172(10):5690–5696. doi: 10.1128/jb.172.10.5690-5696.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed R. R., Winkler H. H. Rickettsia prowazekii and thymidylate metabolism. Growth in thymidylate synthase-deficient eukaryotic host cells. Ann N Y Acad Sci. 1990;590:408–415. doi: 10.1111/j.1749-6632.1990.tb42248.x. [DOI] [PubMed] [Google Scholar]
- WISSEMAN C. L., Jr, JACKSON E. B., HAHN F. E., LEY A. C., SMADEL J. E. Metabolic studies of rickettsiae. I. The effects of antimicrobial substances and enzyme inhibitors on the oxidation of glutamate by purified rickettsiae. J Immunol. 1951 Aug;67(2):123–136. [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Rickettsial hemolysis: rapid method for enumeration of metabolically active typhus rickettsiae. J Clin Microbiol. 1979 May;9(5):645–647. doi: 10.1128/jcm.9.5.645-647.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Newman L. W., Grays R., Green A. E. Metabolism of Rickettsia typhi and Rickettsia akari in irradiated L cells. Infect Immun. 1972 Jul;6(1):50–57. doi: 10.1128/iai.6.1.50-57.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Peterson J. C. Enzymatic activities leading to pyrimidine nucleotide biosynthesis from cell-free extracts of Rickettsia typhi. Infect Immun. 1976 Aug;14(2):439–448. doi: 10.1128/iai.14.2.439-448.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Daugherty R. M. Acquisition of glucose by Rickettsia prowazekii through the nucleotide intermediate uridine 5'-diphosphoglucose. J Bacteriol. 1986 Sep;167(3):805–808. doi: 10.1128/jb.167.3.805-808.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Daugherty R. M. Proline transport and metabolism in Rickettsia prowazekii. J Bacteriol. 1984 May;158(2):460–463. doi: 10.1128/jb.158.2.460-463.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Inhibitory and restorative effects of adenine nucleotides on rickettsial adsorption and hemolysis. Infect Immun. 1974 Jan;9(1):119–126. doi: 10.1128/iai.9.1.119-126.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Potassium permeability of Rickettsia prowazekii. J Bacteriol. 1984 Jan;157(1):197–201. doi: 10.1128/jb.157.1.197-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsial permeability. An ADP-ATP transport system. J Biol Chem. 1976 Jan 25;251(2):389–396. [PubMed] [Google Scholar]
- XEROS N. Deoxyriboside control and synchronization of mitosis. Nature. 1962 May 19;194:682–683. doi: 10.1038/194682a0. [DOI] [PubMed] [Google Scholar]


