Abstract
The tRNA(m5U54)methyltransferase, whose structural gene is designated trmA, catalyzes the formation of 5-methyluridine in position 54 of all tRNA species in Escherichia coli. The synthesis of this enzyme has previously been shown to be both growth rate dependent and stringently regulated, suggesting regulatory features similar to those of rRNA. We have determined the complete nucleotide sequence of the trmA operon in E. coli and the sequence of the trmA promoter region in Salmonella typhimurium and also analyzed the transcriptional regulation of the gene. The trmA and the btuB (encoding the vitamin B12 outer membrane receptor protein) promoters are divergent promoters separated by 102 bp between the transcriptional start sites. The trmA promoters of both E. coli and S. typhimurium share promoter elements with the rRNA P1 promoter. The sequence downstream from the -10 region of the trmA promoter is homologous to the discriminatory region found in stringently regulated promoters. Next to and upstream from the -10 region is a sequence, TCCC, in the trmA promoter that is present in all of the seven rRNA P1 promoters and in some tRNA promoters but not in any other sigma 70 promoter. However, a similar motif is also found in promoters transcribed by the heat shock sigma factor sigma 32. The trmA gene is transcribed as a monocistronic operon, and the 3' end of the transcript is shown to be located downstream from a dyad symmetry region not followed by a poly(U) stretch. Using a trmA-cat operon fusion, we show that the growth rate-dependent regulation of trmA resembles that of rRNA and operates at the level of transcription.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aufrère R., Tempête M., Bohin J. P. Regulation of expression of the gene for vitamin B12 receptor cloned on a multicopy plasmid in Escherichia coli. Mol Gen Genet. 1986 Nov;205(2):358–365. doi: 10.1007/BF00430451. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C. F., Warren R. A. Divergent promoters, a common form of gene organization. Microbiol Rev. 1988 Sep;52(3):318–326. doi: 10.1128/mr.52.3.318-326.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk G. R., Ericson J. U., Gustafsson C. E., Hagervall T. G., Jönsson Y. H., Wikström P. M. Transfer RNA modification. Annu Rev Biochem. 1987;56:263–287. doi: 10.1146/annurev.bi.56.070187.001403. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Carter-Muenchau P., Wolf R. E., Jr Growth-rate-dependent regulation of 6-phosphogluconate dehydrogenase level mediated by an anti-Shine-Dalgarno sequence located within the Escherichia coli gnd structural gene. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1138–1142. doi: 10.1073/pnas.86.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R. H., Lai E., Darnell J. E., Jr Transcriptional control of the mouse prealbumin (transthyretin) gene: both promoter sequences and a distinct enhancer are cell specific. Mol Cell Biol. 1986 Dec;6(12):4697–4708. doi: 10.1128/mcb.6.12.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowing D. W., Bardwell J. C., Craig E. A., Woolford C., Hendrix R. W., Gross C. A. Consensus sequence for Escherichia coli heat shock gene promoters. Proc Natl Acad Sci U S A. 1985 May;82(9):2679–2683. doi: 10.1073/pnas.82.9.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabeel M., Charlier D., Glansdorff N. Studies on the bipolar argECBH operon of E. coli: characterization of restriction endonuclease fragments obtained from gammadargECBH transducing phages and a ColE1 argECBH plasmid. Mol Gen Genet. 1977 Mar 7;151(2):161–168. doi: 10.1007/BF00338690. [DOI] [PubMed] [Google Scholar]
- Dickson R. R., Gaal T., deBoer H. A., deHaseth P. L., Gourse R. L. Identification of promoter mutants defective in growth-rate-dependent regulation of rRNA transcription in Escherichia coli. J Bacteriol. 1989 Sep;171(9):4862–4870. doi: 10.1128/jb.171.9.4862-4870.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürwald H., Hoffmann-Berling H. Endonuclease-I-deficient and ribonuclease I-deficient Escherichia coli mutants. J Mol Biol. 1968 Jul 14;34(2):331–346. doi: 10.1016/0022-2836(68)90257-x. [DOI] [PubMed] [Google Scholar]
- Frey B., McCloskey J., Kersten W., Kersten H. New function of vitamin B12: cobamide-dependent reduction of epoxyqueuosine to queuosine in tRNAs of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1988 May;170(5):2078–2082. doi: 10.1128/jb.170.5.2078-2082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal T., Barkei J., Dickson R. R., deBoer H. A., deHaseth P. L., Alavi H., Gourse R. L. Saturation mutagenesis of an Escherichia coli rRNA promoter and initial characterization of promoter variants. J Bacteriol. 1989 Sep;171(9):4852–4861. doi: 10.1128/jb.171.9.4852-4861.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal T., Gourse R. L. Guanosine 3'-diphosphate 5'-diphosphate is not required for growth rate-dependent control of rRNA synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5533–5537. doi: 10.1073/pnas.87.14.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Reynolds R. P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987 Mar 11;15(5):2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harr R., Fällman P., Häggström M., Wahlström L., Gustafsson P. GENEUS, a computer system for DNA and protein sequence analysis containing an information retrieval system for the EMBL data library. Nucleic Acids Res. 1986 Jan 10;14(1):273–284. doi: 10.1093/nar/14.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K., Kadner R. J. Nucleotide sequence of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J Bacteriol. 1985 Mar;161(3):904–908. doi: 10.1128/jb.161.3.904-908.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner P., Arber W. Mutational analysis of a prokaryotic recombinational enhancer element with two functions. EMBO J. 1989 Feb;8(2):577–585. doi: 10.1002/j.1460-2075.1989.tb03412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josaitis C. A., Gaal T., Ross W., Gourse R. L. Sequences upstream of the-35 hexamer of rrnB P1 affect promoter strength and upstream activation. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):307–311. doi: 10.1016/0167-4781(90)90186-6. [DOI] [PubMed] [Google Scholar]
- Kadner R. J. Repression of synthesis of the vitamin B12 receptor in Escherichia coli. J Bacteriol. 1978 Dec;136(3):1050–1057. doi: 10.1128/jb.136.3.1050-1057.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimasauskas S., Timinskas A., Menkevicius S., Butkienè D., Butkus V., Janulaitis A. Sequence motifs characteristic of DNA[cytosine-N4]methyltransferases: similarity to adenine and cytosine-C5 DNA-methylases. Nucleic Acids Res. 1989 Dec 11;17(23):9823–9832. doi: 10.1093/nar/17.23.9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotsky R. A., Schwartz I. Measurement of cat expression from growth-rate-regulated promoters employing beta-lactamase activity as an indicator of plasmid copy number. Gene. 1987;55(1):141–146. doi: 10.1016/0378-1119(87)90257-5. [DOI] [PubMed] [Google Scholar]
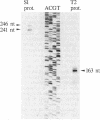
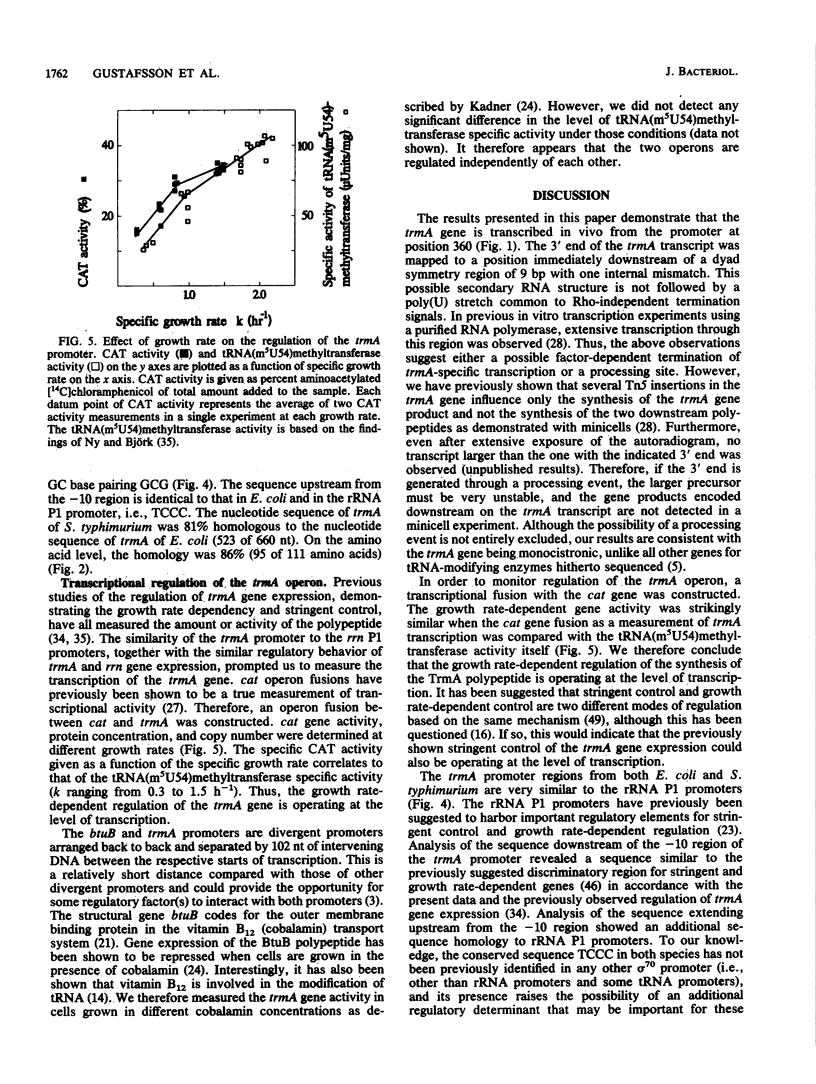
- Lindström P. H., Stüber D., Björk G. R. Genetic organization and transcription from the gene (trmA) responsible for synthesis of tRNA (uracil-5)-methyltransferase by Escherichia coli. J Bacteriol. 1985 Dec;164(3):1117–1123. doi: 10.1128/jb.164.3.1117-1123.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L., Vanet A., Vijgenboom E., Bosch L. The role of FIS in trans activation of stable RNA operons of E. coli. EMBO J. 1990 Mar;9(3):727–734. doi: 10.1002/j.1460-2075.1990.tb08166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Ny T., Björk G. R. Cloning and restriction mapping of the trmA gene coding for transfer ribonucleic acid (5-methyluridine)-methyltransferase in Escherichia coli K-12. J Bacteriol. 1980 May;142(2):371–379. doi: 10.1128/jb.142.2.371-379.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny T., Björk G. R. Growth rate-dependent regulation of transfer ribonucleic acid (5-methyluridine) methyltransferase in Escherichia coli B/r. J Bacteriol. 1980 Jan;141(1):67–73. doi: 10.1128/jb.141.1.67-73.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny T., Björk G. R. Stringent regulation of the synthesis of a transfer ribonucleic acid biosynthetic enzyme: transfer ribonucleic acid(m5U)methyltransferase from Escherichia coli. J Bacteriol. 1977 May;130(2):635–641. doi: 10.1128/jb.130.2.635-641.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny T., Lindström H. R., Hagervall T. G., Björk G. R. Purification of transfer RNA (m5U54)-methyltransferase from Escherichia coli. Association with RNA. Eur J Biochem. 1988 Nov 15;177(3):467–475. doi: 10.1111/j.1432-1033.1988.tb14396.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schickor P., Metzger W., Werel W., Lederer H., Heumann H. Topography of intermediates in transcription initiation of E.coli. EMBO J. 1990 Jul;9(7):2215–2220. doi: 10.1002/j.1460-2075.1990.tb07391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker N. G., Fairweather N. F., Spratt B. G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982 Jun;18(3):335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Studnicka G. M. Nucleotide sequence homologies in control regions of prokaryotic genomes. Gene. 1987;58(1):45–57. doi: 10.1016/0378-1119(87)90028-x. [DOI] [PubMed] [Google Scholar]
- Tao K., Makino K., Yonei S., Nakata A., Shinagawa H. Molecular cloning and nucleotide sequencing of oxyR, the positive regulatory gene of a regulon for an adaptive response to oxidative stress in Escherichia coli: homologies between OxyR protein and a family of bacterial activator proteins. Mol Gen Genet. 1989 Sep;218(3):371–376. doi: 10.1007/BF00332397. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Brose E. C. Modified Birnboim-Doly method for rapid detection of plasmid copy number. Nucleic Acids Res. 1988 Sep 26;16(18):9056–9056. doi: 10.1093/nar/16.18.9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A. Conserved features of coordinately regulated E. coli promoters. Nucleic Acids Res. 1984 Mar 26;12(6):2605–2618. doi: 10.1093/nar/12.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias M., Göringer H. U., Wagner R. Influence of the GCGC discriminator motif introduced into the ribosomal RNA P2- and tac promoter on growth-rate control and stringent sensitivity. EMBO J. 1989 Nov;8(11):3357–3363. doi: 10.1002/j.1460-2075.1989.tb08498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]