Abstract
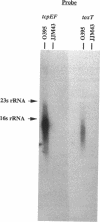
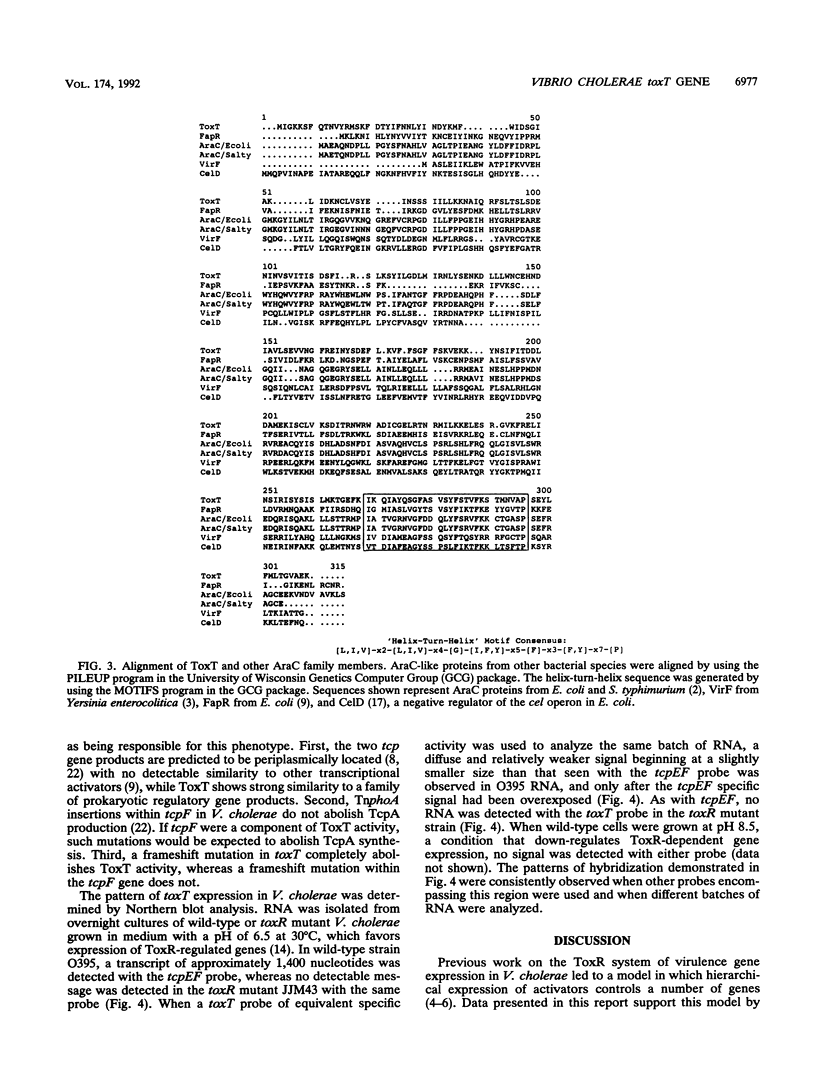
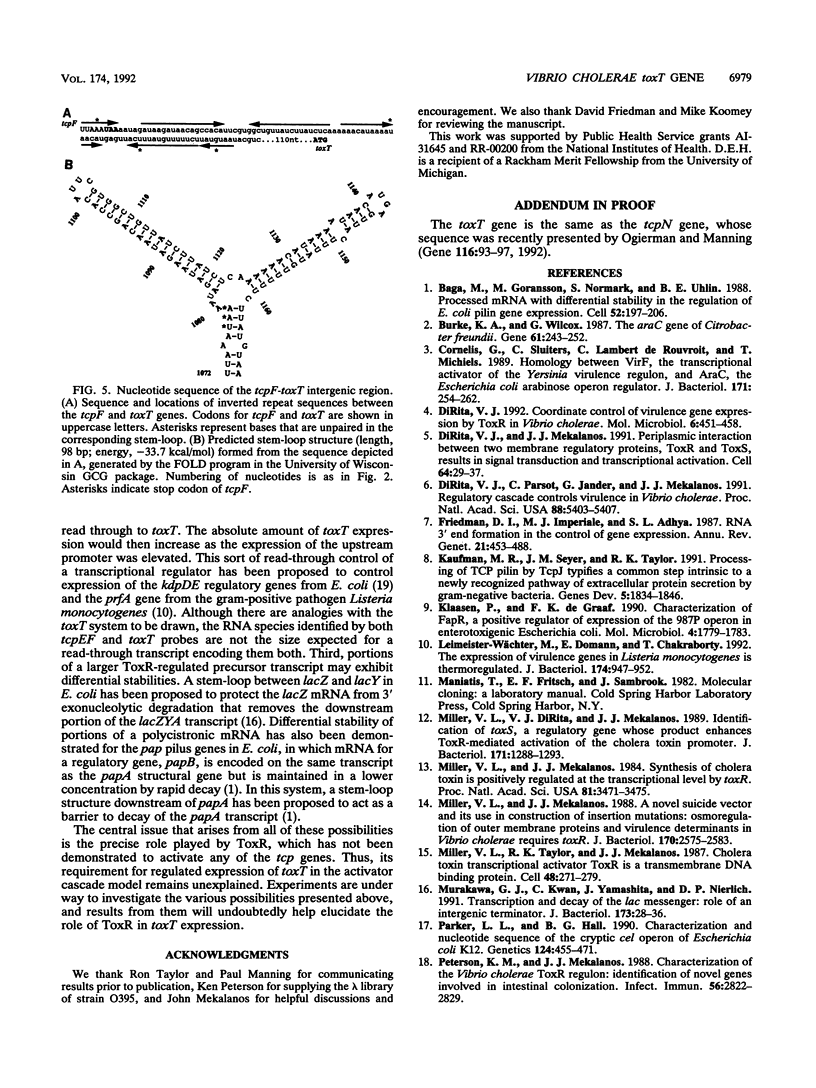
Virulence gene expression in Vibrio cholerae is postulated to involve ToxR-dependent activation of the toxT gene followed by ToxT activation of virulence genes, including several of those involved in biogenesis of the toxin-coregulated pilus. ToxR is a transmembrane, DNA-binding protein which is a member of the OmpR subclass of two-component activator systems in bacteria. Data presented in this report demonstrate that ToxT is similar to the AraC family of transcriptional activators identified in a variety of gram-negative bacteria. The toxT open reading frame begins approximately 200 nucleotides from the end of the tcpF gene, which is part of a cluster of genes responsible for production of the toxin-coregulated pilus. Accumulation of toxT specific mRNA is ToxR dependent and is modulated by environmental conditions that modulate expression of the regulon. Within the intergenic region between tcpF and toxT is a potential stem-loop structure of an unusual nature which may play a role in regulating expression of toxT mRNA. Experiments with tcpF and toxT cloned behind a strong, constitutive promoter suggest that the two genes can be cotranscribed, but Northern (RNA) blot analysis of V. cholerae suggests that if they are, steady-state levels of their messages may be controlled by a posttranscriptional mechanism. Possible mechanisms for ToxR-dependent expression of toxT are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke K. A., Wilcox G. The araC gene of Citrobacter freundii. Gene. 1987;61(3):243–252. doi: 10.1016/0378-1119(87)90188-0. [DOI] [PubMed] [Google Scholar]
- Båga M., Göransson M., Normark S., Uhlin B. E. Processed mRNA with differential stability in the regulation of E. coli pilin gene expression. Cell. 1988 Jan 29;52(2):197–206. doi: 10.1016/0092-8674(88)90508-9. [DOI] [PubMed] [Google Scholar]
- Cornelis G., Sluiters C., de Rouvroit C. L., Michiels T. Homology between virF, the transcriptional activator of the Yersinia virulence regulon, and AraC, the Escherichia coli arabinose operon regulator. J Bacteriol. 1989 Jan;171(1):254–262. doi: 10.1128/jb.171.1.254-262.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRita V. J. Co-ordinate expression of virulence genes by ToxR in Vibrio cholerae. Mol Microbiol. 1992 Feb;6(4):451–458. doi: 10.1111/j.1365-2958.1992.tb01489.x. [DOI] [PubMed] [Google Scholar]
- DiRita V. J., Mekalanos J. J. Periplasmic interaction between two membrane regulatory proteins, ToxR and ToxS, results in signal transduction and transcriptional activation. Cell. 1991 Jan 11;64(1):29–37. doi: 10.1016/0092-8674(91)90206-e. [DOI] [PubMed] [Google Scholar]
- DiRita V. J., Parsot C., Jander G., Mekalanos J. J. Regulatory cascade controls virulence in Vibrio cholerae. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5403–5407. doi: 10.1073/pnas.88.12.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. I., Imperiale M. J., Adhya S. L. RNA 3' end formation in the control of gene expression. Annu Rev Genet. 1987;21:453–488. doi: 10.1146/annurev.ge.21.120187.002321. [DOI] [PubMed] [Google Scholar]
- Kaufman M. R., Seyer J. M., Taylor R. K. Processing of TCP pilin by TcpJ typifies a common step intrinsic to a newly recognized pathway of extracellular protein secretion by gram-negative bacteria. Genes Dev. 1991 Oct;5(10):1834–1846. doi: 10.1101/gad.5.10.1834. [DOI] [PubMed] [Google Scholar]
- Klaasen P., de Graaf F. K. Characterization of FapR, a positive regulator of expression of the 987P operon in enterotoxigenic Escherichia coli. Mol Microbiol. 1990 Oct;4(10):1779–1783. doi: 10.1111/j.1365-2958.1990.tb00556.x. [DOI] [PubMed] [Google Scholar]
- Leimeister-Wächter M., Domann E., Chakraborty T. The expression of virulence genes in Listeria monocytogenes is thermoregulated. J Bacteriol. 1992 Feb;174(3):947–952. doi: 10.1128/jb.174.3.947-952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., DiRita V. J., Mekalanos J. J. Identification of toxS, a regulatory gene whose product enhances toxR-mediated activation of the cholera toxin promoter. J Bacteriol. 1989 Mar;171(3):1288–1293. doi: 10.1128/jb.171.3.1288-1293.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988 Jun;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. Synthesis of cholera toxin is positively regulated at the transcriptional level by toxR. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3471–3475. doi: 10.1073/pnas.81.11.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Taylor R. K., Mekalanos J. J. Cholera toxin transcriptional activator toxR is a transmembrane DNA binding protein. Cell. 1987 Jan 30;48(2):271–279. doi: 10.1016/0092-8674(87)90430-2. [DOI] [PubMed] [Google Scholar]
- Murakawa G. J., Kwan C., Yamashita J., Nierlich D. P. Transcription and decay of the lac messenger: role of an intergenic terminator. J Bacteriol. 1991 Jan;173(1):28–36. doi: 10.1128/jb.173.1.28-36.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogierman M. A., Manning P. A. Homology of TcpN, a putative regulatory protein of Vibrio cholerae, to the AraC family of transcriptional activators. Gene. 1992 Jul 1;116(1):93–97. doi: 10.1016/0378-1119(92)90634-2. [DOI] [PubMed] [Google Scholar]
- Parker L. L., Hall B. G. Characterization and nucleotide sequence of the cryptic cel operon of Escherichia coli K12. Genetics. 1990 Mar;124(3):455–471. doi: 10.1093/genetics/124.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. M., Mekalanos J. J. Characterization of the Vibrio cholerae ToxR regulon: identification of novel genes involved in intestinal colonization. Infect Immun. 1988 Nov;56(11):2822–2829. doi: 10.1128/iai.56.11.2822-2829.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polarek J. W., Williams G., Epstein W. The products of the kdpDE operon are required for expression of the Kdp ATPase of Escherichia coli. J Bacteriol. 1992 Apr;174(7):2145–2151. doi: 10.1128/jb.174.7.2145-2151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J. L., Rojo F., Zhou L., Timmis K. N. A family of positive regulators related to the Pseudomonas putida TOL plasmid XylS and the Escherichia coli AraC activators. Nucleic Acids Res. 1990 Apr 25;18(8):2149–2152. doi: 10.1093/nar/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Weiss B. Two divergently transcribed genes, soxR and soxS, control a superoxide response regulon of Escherichia coli. J Bacteriol. 1991 May;173(9):2864–2871. doi: 10.1128/jb.173.9.2864-2871.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]