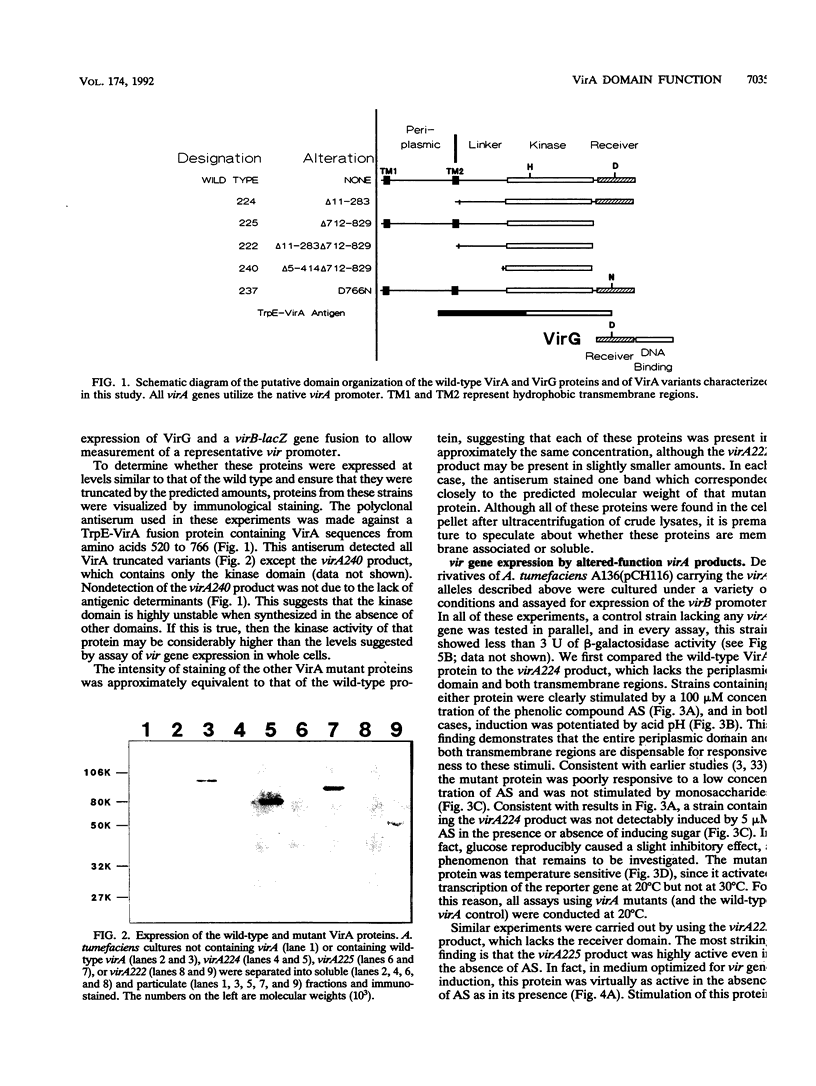
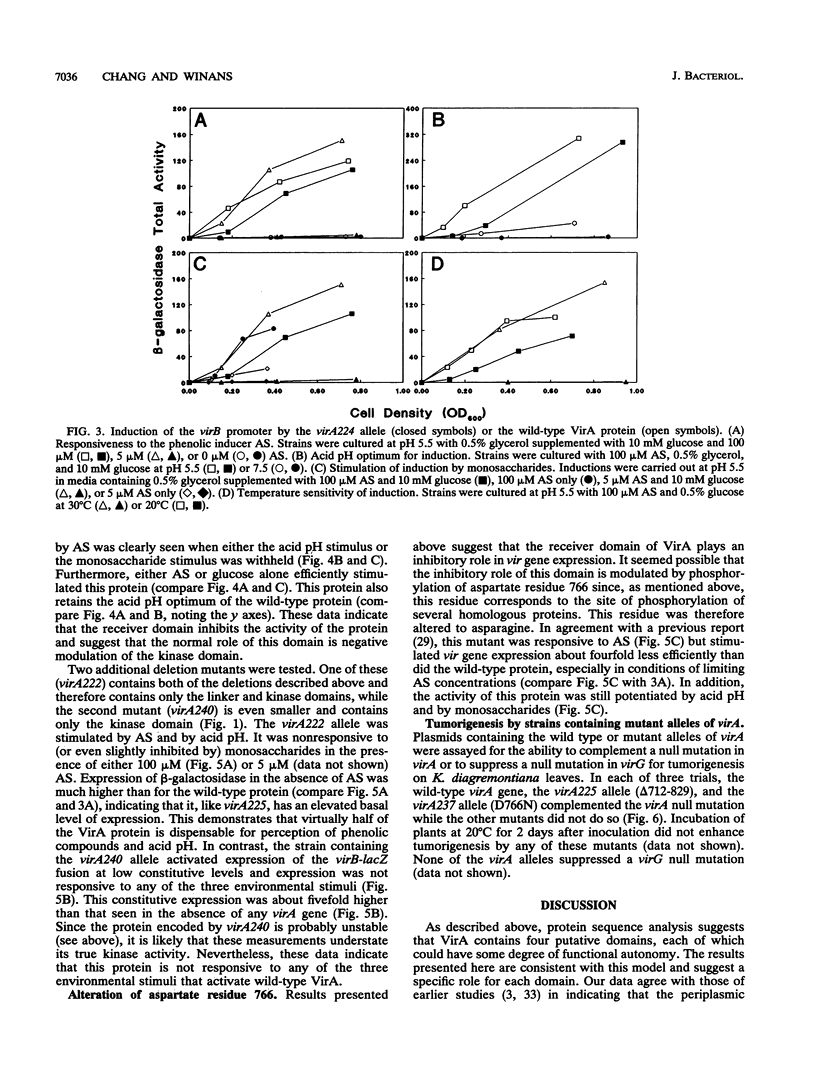
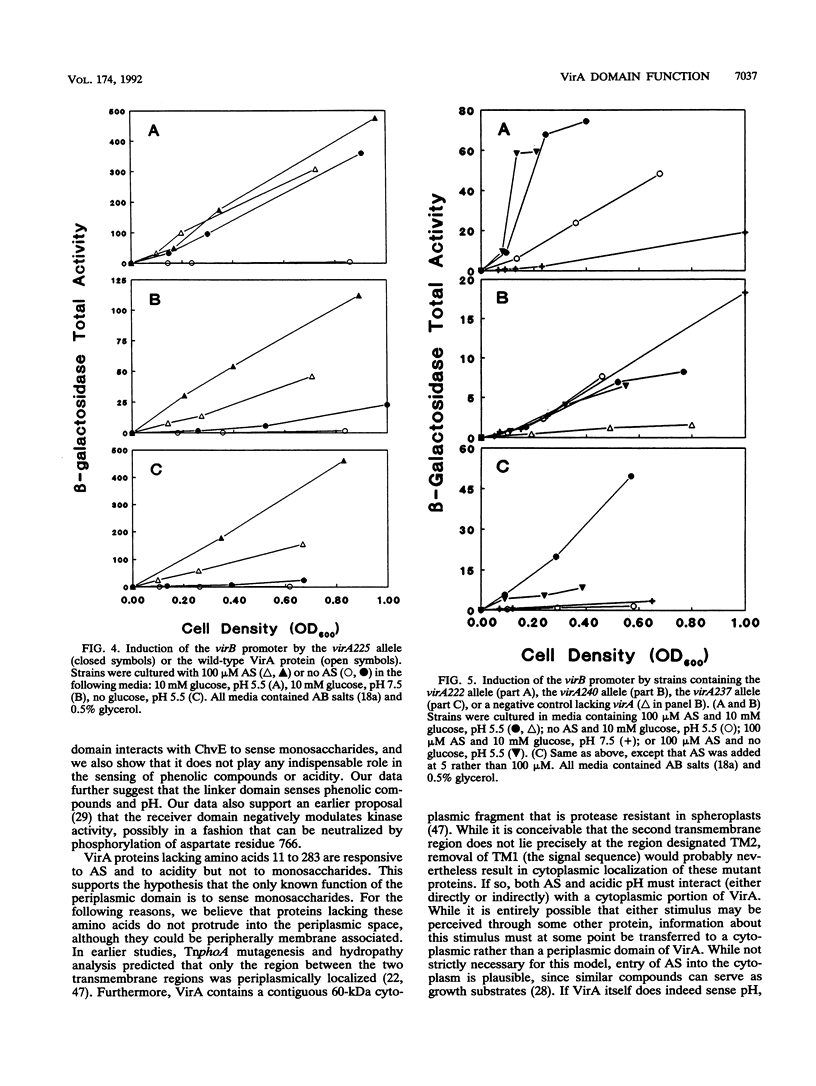
Abstract
VirA and VirG activate the Agrobacterium tumefaciens vir regulon in response to phenolic compounds, monosaccharides, and acidity released from plant wound sites. VirA contains an amino-terminal periplasmic domain and three cytoplasmic domains: a linker, a protein kinase, and a phosphoryl receiver. We constructed internal deletions of virA that truncate one or more domains and tested the ability of the resulting proteins to mediate environmentally responsive vir gene activation in vivo. The periplasmic domain is required for sensing of monosaccharides (in agreement with earlier results), while the linker domain is required for sensing of phenolic compounds and acidity. The phosphoryl receiver domain of VirA plays an inhibitory role in signal transduction that may be modulated by phosphorylation. The carboxy terminus of the protein was also dispensable for tumorigenesis, while the periplasmic domain was required.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ankenbauer R. G., Best E. A., Palanca C. A., Nester E. W. Mutants of the Agrobacterium tumefaciens virA gene exhibiting acetosyringone-independent expression of the vir regulon. Mol Plant Microbe Interact. 1991 Jul-Aug;4(4):400–406. doi: 10.1094/mpmi-4-400. [DOI] [PubMed] [Google Scholar]
- Aricó B., Miller J. F., Roy C., Stibitz S., Monack D., Falkow S., Gross R., Rappuoli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangelosi G. A., Ankenbauer R. G., Nester E. W. Sugars induce the Agrobacterium virulence genes through a periplasmic binding protein and a transmembrane signal protein. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6708–6712. doi: 10.1073/pnas.87.17.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Winans S. C. Controlled expression of the transcriptional activator gene virG in Agrobacterium tumefaciens by using the Escherichia coli lac promoter. J Bacteriol. 1991 Feb;173(3):1139–1144. doi: 10.1128/jb.173.3.1139-1144.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close T. J., Zaitlin D., Kado C. I. Design and development of amplifiable broad-host-range cloning vectors: analysis of the vir region of Agrobacterium tumefaciens plasmid pTiC58. Plasmid. 1984 Sep;12(2):111–118. doi: 10.1016/0147-619x(84)90057-x. [DOI] [PubMed] [Google Scholar]
- Das A., Pazour G. J. Delineation of the regulatory region sequences of Agrobacterium tumefaciens virB operon. Nucleic Acids Res. 1989 Jun 26;17(12):4541–4550. doi: 10.1093/nar/17.12.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabak E. M., Willis D. K. The lemA gene required for pathogenicity of Pseudomonas syringae pv. syringae on bean is a member of a family of two-component regulators. J Bacteriol. 1992 May;174(9):3011–3020. doi: 10.1128/jb.174.9.3011-3020.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Iuchi S., Matsuda Z., Fujiwara T., Lin E. C. The arcB gene of Escherichia coli encodes a sensor-regulator protein for anaerobic repression of the arc modulon. Mol Microbiol. 1990 May;4(5):715–727. doi: 10.1111/j.1365-2958.1990.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Jin S. G., Prusti R. K., Roitsch T., Ankenbauer R. G., Nester E. W. Phosphorylation of the VirG protein of Agrobacterium tumefaciens by the autophosphorylated VirA protein: essential role in biological activity of VirG. J Bacteriol. 1990 Sep;172(9):4945–4950. doi: 10.1128/jb.172.9.4945-4950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S. G., Roitsch T., Christie P. J., Nester E. W. The regulatory VirG protein specifically binds to a cis-acting regulatory sequence involved in transcriptional activation of Agrobacterium tumefaciens virulence genes. J Bacteriol. 1990 Feb;172(2):531–537. doi: 10.1128/jb.172.2.531-537.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Roitsch T., Ankenbauer R. G., Gordon M. P., Nester E. W. The VirA protein of Agrobacterium tumefaciens is autophosphorylated and is essential for vir gene regulation. J Bacteriol. 1990 Feb;172(2):525–530. doi: 10.1128/jb.172.2.525-530.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaie K., Dull T. J., Graf T., Schlessinger J., Ullrich A., Beug H., Vennström B. Truncation of the human EGF receptor leads to differential transforming potentials in primary avian fibroblasts and erythroblasts. EMBO J. 1988 Oct;7(10):3061–3071. doi: 10.1002/j.1460-2075.1988.tb03171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofoid E. C., Parkinson J. S. Tandem translation starts in the cheA locus of Escherichia coli. J Bacteriol. 1991 Mar;173(6):2116–2119. doi: 10.1128/jb.173.6.2116-2119.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leroux B., Yanofsky M. F., Winans S. C., Ward J. E., Ziegler S. F., Nester E. W. Characterization of the virA locus of Agrobacterium tumefaciens: a transcriptional regulator and host range determinant. EMBO J. 1987 Apr;6(4):849–856. doi: 10.1002/j.1460-2075.1987.tb04830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B. L., Lax I., Kris R., Dombalagian M., Honegger A. M., Howk R., Givol D., Ullrich A., Schlessinger J. All autophosphorylation sites of epidermal growth factor (EGF) receptor and HER2/neu are located in their carboxyl-terminal tails. Identification of a novel site in EGF receptor. J Biol Chem. 1989 Jun 25;264(18):10667–10671. [PubMed] [Google Scholar]
- McCleary W. R., Zusman D. R. FrzE of Myxococcus xanthus is homologous to both CheA and CheY of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5898–5902. doi: 10.1073/pnas.87.15.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers L. S., Regensburg-Tuïnk T. J., Bourret R. B., Sedee N. J., Schilperoort R. A., Hooykaas P. J. Membrane topology and functional analysis of the sensory protein VirA of Agrobacterium tumefaciens. EMBO J. 1989 Jul;8(7):1919–1925. doi: 10.1002/j.1460-2075.1989.tb03595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers L. S., Thompson D. V., Idler K. B., Schilperoort R. A., Hooykaas P. J. Nucleotide sequence of the virulence gene virG of the Agrobacterium tumefaciens octopine Ti plasmid: significant homology between virG and the regulatory genes ompR, phoB and dye of E. coli. Nucleic Acids Res. 1986 Dec 22;14(24):9933–9942. doi: 10.1093/nar/14.24.9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel P., Powell B. S., Rogowsky P. M., Kado C. I. Characterization of the virA virulence gene of the nopaline plasmid, pTiC58, of Agrobacterium tumefaciens. Mol Microbiol. 1989 Sep;3(9):1237–1246. doi: 10.1111/j.1365-2958.1989.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Nagasawa S., Tokishita S., Aiba H., Mizuno T. A novel sensor-regulator protein that belongs to the homologous family of signal-transduction proteins involved in adaptive responses in Escherichia coli. Mol Microbiol. 1992 Mar;6(6):799–807. doi: 10.1111/j.1365-2958.1992.tb01530.x. [DOI] [PubMed] [Google Scholar]
- Ninfa A. J. Protein phosphorylation and the regulation of cellular processes by the homologous two-component regulatory systems of bacteria. Genet Eng (N Y) 1991;13:39–72. doi: 10.1007/978-1-4615-3760-1_2. [DOI] [PubMed] [Google Scholar]
- Parke D., Ornston L. N., Nester E. W. Chemotaxis to plant phenolic inducers of virulence genes is constitutively expressed in the absence of the Ti plasmid in Agrobacterium tumefaciens. J Bacteriol. 1987 Nov;169(11):5336–5338. doi: 10.1128/jb.169.11.5336-5338.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G. J., Ta C. N., Das A. Mutants of Agrobacterium tumefaciens with elevated vir gene expression. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6941–6945. doi: 10.1073/pnas.88.16.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell B. S., Powell G. K., Morris R. O., Rogowsky P. M., Kado C. I. Nucleotide sequence of the virG locus of the Agrobacterium tumefaciens plasmid pTiC58. Mol Microbiol. 1987 Nov;1(3):309–316. doi: 10.1111/j.1365-2958.1987.tb01937.x. [DOI] [PubMed] [Google Scholar]
- Roitsch T., Wang H., Jin S. G., Nester E. W. Mutational analysis of the VirG protein, a transcriptional activator of Agrobacterium tumefaciens virulence genes. J Bacteriol. 1990 Oct;172(10):6054–6060. doi: 10.1128/jb.172.10.6054-6060.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda N., Toyoda-Yamamoto A., Nagamine J., Usami S., Katayama M., Sakagami Y., Machida Y. Control of expression of Agrobacterium vir genes by synergistic actions of phenolic signal molecules and monosaccharides. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6684–6688. doi: 10.1073/pnas.87.17.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonczewski J. L., Gonzalez T. N., Bartholomew F. M., Holt N. J. Mu d-directed lacZ fusions regulated by low pH in Escherichia coli. J Bacteriol. 1987 Jul;169(7):3001–3006. doi: 10.1128/jb.169.7.3001-3006.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Nester E. W. The genetic and transcriptional organization of the vir region of the A6 Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1986 Jul;5(7):1445–1454. doi: 10.1002/j.1460-2075.1986.tb04381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Nester E. W., Zambryski P. C. A plant cell factor induces Agrobacterium tumefaciens vir gene expression. Proc Natl Acad Sci U S A. 1986 Jan;83(2):379–383. doi: 10.1073/pnas.83.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Zambryski P. C. virA and virG control the plant-induced activation of the T-DNA transfer process of A. tumefaciens. Cell. 1986 Aug 1;46(3):325–333. doi: 10.1016/0092-8674(86)90653-7. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout V., Gottesman S. RcsB and RcsC: a two-component regulator of capsule synthesis in Escherichia coli. J Bacteriol. 1990 Feb;172(2):659–669. doi: 10.1128/jb.172.2.659-669.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- White S., Tuttle F. E., Blankenhorn D., Dosch D. C., Slonczewski J. L. pH dependence and gene structure of inaA in Escherichia coli. J Bacteriol. 1992 Mar;174(5):1537–1543. doi: 10.1128/jb.174.5.1537-1543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Ebert P. R., Stachel S. E., Gordon M. P., Nester E. W. A gene essential for Agrobacterium virulence is homologous to a family of positive regulatory loci. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8278–8282. doi: 10.1073/pnas.83.21.8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Kerstetter R. A., Ward J. E., Nester E. W. A protein required for transcriptional regulation of Agrobacterium virulence genes spans the cytoplasmic membrane. J Bacteriol. 1989 Mar;171(3):1616–1622. doi: 10.1128/jb.171.3.1616-1622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C. Two-way chemical signaling in Agrobacterium-plant interactions. Microbiol Rev. 1992 Mar;56(1):12–31. doi: 10.1128/mr.56.1.12-31.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]