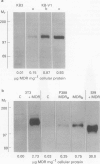
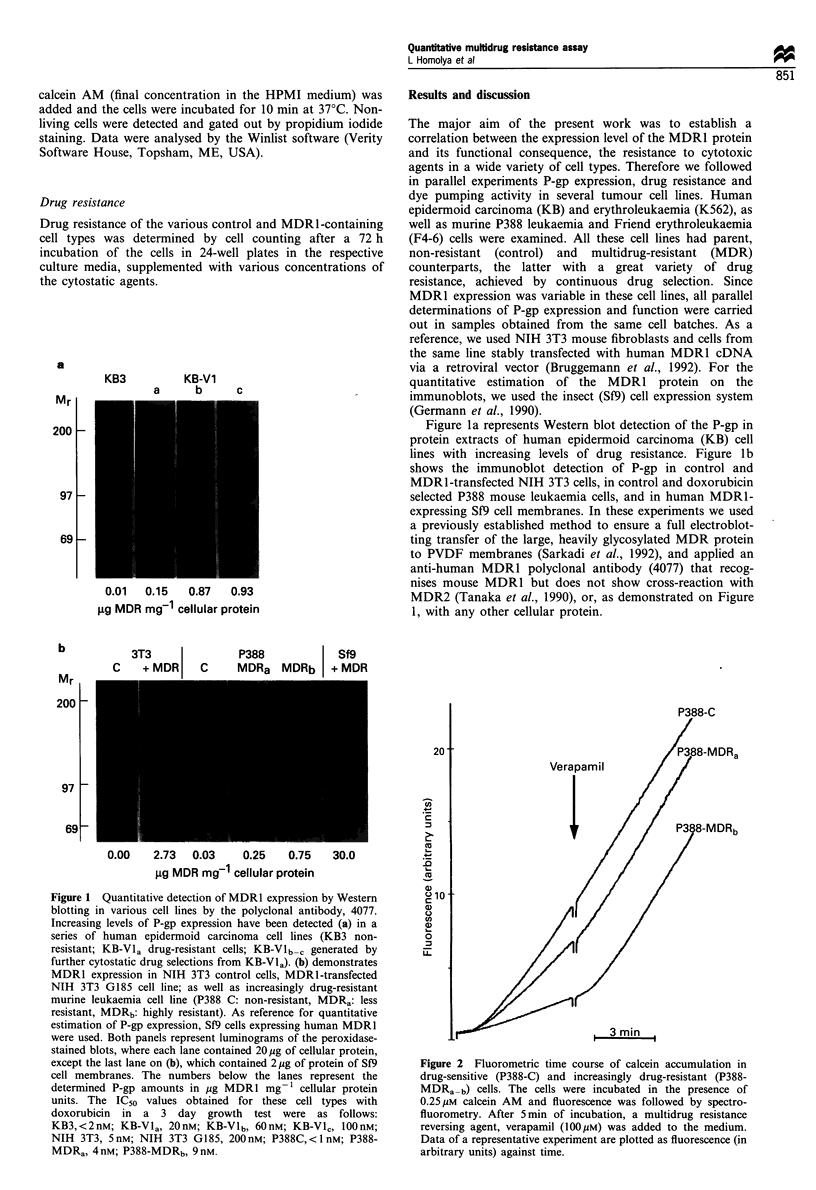
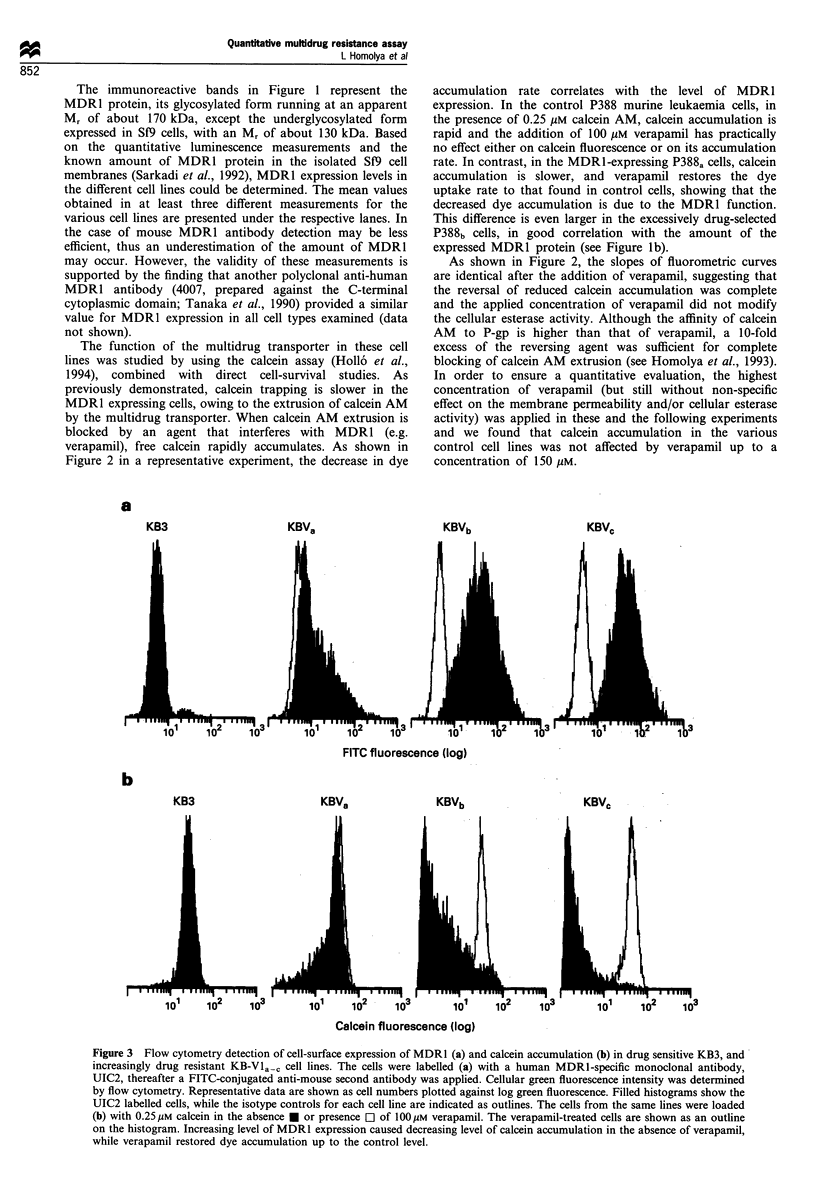
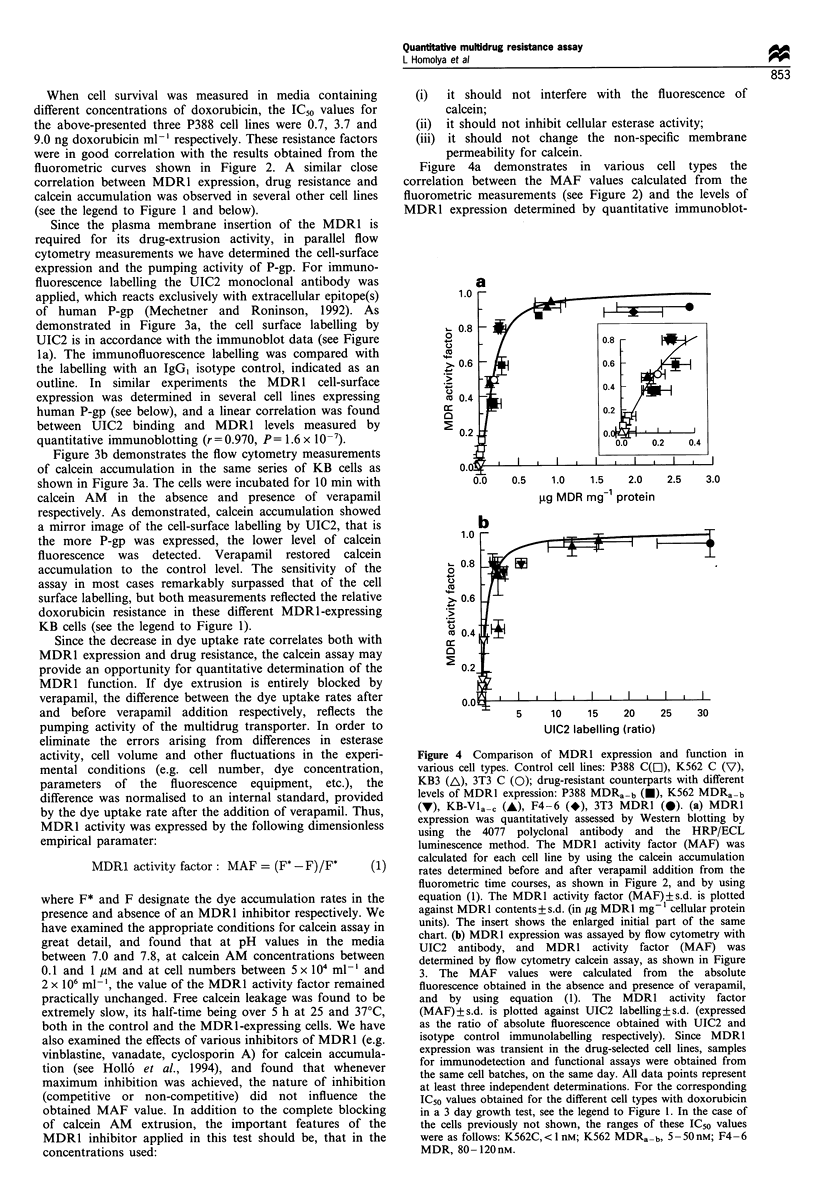
Abstract
A rapid, functional and quantitative diagnostic method for the estimation of the P-glycoprotein (P-gp)-dependent multidrug resistance is required in the clinical treatment of human tumours, as chemotherapy protocols and resistance-reversing agents could be applied accordingly. In the present work, by using a calcein accumulation method in combination with immunorecognition and drug-resistance studies, a new method is described for the quantitative estimation of the expression and function of the multidrug transporter. MDR1-transfected and drug-selected tumour cell lines with various levels of drug resistance were examined. The expression of P-gp and its cell-surface appearance were assessed by quantitative immunoblotting and by immunofluorescence cytometry. The transport function of the P-gp was assessed by measuring the extrusion of calcein acetoxymethyl ester (AM) with fluorometry and flow cytometry, while in parallel experiments drug resistance was directly examined in cell survival assays. The MDR1 activity factor (MAF), calculated from the calcein AM extrusion assay, is demonstrated to provide a reliable quantitative measure for MDR1 specific activity, reflecting cellular drug resistance. This relatively simple and rapid new functional P-gp assay surpasses the formerly used techniques in both sensitivity and reproducibility.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenberg G. A., Vanoye C. G., Horton J. K., Reuss L. Unidirectional fluxes of rhodamine 123 in multidrug-resistant cells: evidence against direct drug extrusion from the plasma membrane. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4654–4657. doi: 10.1073/pnas.91.11.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornmann W. G., Roepe P. D. Analysis of drug transport kinetics in multidrug-resistant cells using a novel coumarin-vinblastine compound. Biochemistry. 1994 Oct 25;33(42):12665–12675. doi: 10.1021/bi00208a018. [DOI] [PubMed] [Google Scholar]
- Bruggemann E. P., Currier S. J., Gottesman M. M., Pastan I. Characterization of the azidopine and vinblastine binding site of P-glycoprotein. J Biol Chem. 1992 Oct 15;267(29):21020–21026. [PubMed] [Google Scholar]
- Chaudhary P. M., Roninson I. B. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell. 1991 Jul 12;66(1):85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992 Dec 4;258(5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Feller N., Broxterman H. J., Währer D. C., Pinedo H. M. ATP-dependent efflux of calcein by the multidrug resistance protein (MRP): no inhibition by intracellular glutathione depletion. FEBS Lett. 1995 Jul 17;368(2):385–388. doi: 10.1016/0014-5793(95)00677-2. [DOI] [PubMed] [Google Scholar]
- Feller N., Kuiper C. M., Lankelma J., Ruhdal J. K., Scheper R. J., Pinedo H. M., Broxterman H. J. Functional detection of MDR1/P170 and MRP/P190-mediated multidrug resistance in tumour cells by flow cytometry. Br J Cancer. 1995 Sep;72(3):543–549. doi: 10.1038/bjc.1995.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey T., Yue S., Haugland R. P. Dyes providing increased sensitivity in flow-cytometric dye-efflux assays for multidrug resistance. Cytometry. 1995 Jul 1;20(3):218–227. doi: 10.1002/cyto.990200305. [DOI] [PubMed] [Google Scholar]
- Germann U. A., Willingham M. C., Pastan I., Gottesman M. M. Expression of the human multidrug transporter in insect cells by a recombinant baculovirus. Biochemistry. 1990 Mar 6;29(9):2295–2303. doi: 10.1021/bi00461a013. [DOI] [PubMed] [Google Scholar]
- Goldstein L. J., Pastan I., Gottesman M. M. Multidrug resistance in human cancer. Crit Rev Oncol Hematol. 1992;12(3):243–253. doi: 10.1016/1040-8428(92)90057-w. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Herweijer H., van den Engh G., Nooter K. A rapid and sensitive flow cytometric method for the detection of multidrug-resistant cells. Cytometry. 1989 Jul;10(4):463–468. doi: 10.1002/cyto.990100415. [DOI] [PubMed] [Google Scholar]
- Holló Z., Homolya L., Davis C. W., Sarkadi B. Calcein accumulation as a fluorometric functional assay of the multidrug transporter. Biochim Biophys Acta. 1994 May 11;1191(2):384–388. doi: 10.1016/0005-2736(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Homolya L., Holló Z., Germann U. A., Pastan I., Gottesman M. M., Sarkadi B. Fluorescent cellular indicators are extruded by the multidrug resistance protein. J Biol Chem. 1993 Oct 15;268(29):21493–21496. [PubMed] [Google Scholar]
- Lee J. S., Paull K., Alvarez M., Hose C., Monks A., Grever M., Fojo A. T., Bates S. E. Rhodamine efflux patterns predict P-glycoprotein substrates in the National Cancer Institute drug screen. Mol Pharmacol. 1994 Oct;46(4):627–638. [PubMed] [Google Scholar]
- Lelong I. H., Guzikowski A. P., Haugland R. P., Pastan I., Gottesman M. M., Willingham M. C. Fluorescent verapamil derivative for monitoring activity of the multidrug transporter. Mol Pharmacol. 1991 Oct;40(4):490–494. [PubMed] [Google Scholar]
- Mechetner E. B., Roninson I. B. Efficient inhibition of P-glycoprotein-mediated multidrug resistance with a monoclonal antibody. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5824–5828. doi: 10.1073/pnas.89.13.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepe P. D. Analysis of the steady-state and initial rate of doxorubicin efflux from a series of multidrug-resistant cells expressing different levels of P-glycoprotein. Biochemistry. 1992 Dec 22;31(50):12555–12564. doi: 10.1021/bi00165a003. [DOI] [PubMed] [Google Scholar]
- Roepe P. D., Wei L. Y., Cruz J., Carlson D. Lower electrical membrane potential and altered pHi homeostasis in multidrug-resistant (MDR) cells: further characterization of a series of MDR cell lines expressing different levels of P-glycoprotein. Biochemistry. 1993 Oct 19;32(41):11042–11056. doi: 10.1021/bi00092a014. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Price E. M., Boucher R. C., Germann U. A., Scarborough G. A. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J Biol Chem. 1992 Mar 5;267(7):4854–4858. [PubMed] [Google Scholar]
- Spoelstra E. C., Westerhoff H. V., Dekker H., Lankelma J. Kinetics of daunorubicin transport by P-glycoprotein of intact cancer cells. Eur J Biochem. 1992 Jul 15;207(2):567–579. doi: 10.1111/j.1432-1033.1992.tb17083.x. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Currier S. J., Bruggemann E. P., Ueda K., Germann U. A., Pastan I., Gottesman M. M. Use of recombinant P-glycoprotein fragments to produce antibodies to the multidrug transporter. Biochem Biophys Res Commun. 1990 Jan 15;166(1):180–186. doi: 10.1016/0006-291x(90)91928-l. [DOI] [PubMed] [Google Scholar]
- Thiebaut F., Currier S. J., Whitaker J., Haugland R. P., Gottesman M. M., Pastan I., Willingham M. C. Activity of the multidrug transporter results in alkalinization of the cytosol: measurement of cytosolic pH by microinjection of a pH-sensitive dye. J Histochem Cytochem. 1990 May;38(5):685–690. doi: 10.1177/38.5.1692055. [DOI] [PubMed] [Google Scholar]
- Wall D. M., Hu X. F., Zalcberg J. R., Parkin J. D. Rapid functional assay for multidrug resistance in human tumor cell lines using the fluorescent indicator fluo-3. J Natl Cancer Inst. 1991 Feb 6;83(3):206–207. doi: 10.1093/jnci/83.3.206. [DOI] [PubMed] [Google Scholar]
- Wall D. M., Sparrow R., Hu X. F., Nadalin G., Zalcberg J. R., Marschner I. C., Van der Weyden M., Parkin J. D. Clinical application of a rapid, functional assay for multidrug resistance based on accumulation of the fluorescent dye, fluo-3. Eur J Cancer. 1993;29A(7):1024–1027. doi: 10.1016/s0959-8049(05)80216-3. [DOI] [PubMed] [Google Scholar]
- Weaver J. L., Pine P. S., Aszalos A., Schoenlein P. V., Currier S. J., Padmanabhan R., Gottesman M. M. Laser scanning and confocal microscopy of daunorubicin, doxorubicin, and rhodamine 123 in multidrug-resistant cells. Exp Cell Res. 1991 Oct;196(2):323–329. doi: 10.1016/0014-4827(91)90267-x. [DOI] [PubMed] [Google Scholar]