Abstract
SDZ PSC 833 (PSC 833) is a non-immunosuppressive analogue of cyclosporin A and is a potent modifier of P-glycoprotein (P-gp)-mediated multidrug resistance. The present study was undertaken to evaluate whether doxorubicin (DOX) pharmacokinetic and anti-tumour activity on P388- and P388/DOX-resistant leukaemia was modified by PSC 833 pretreatment. P388- or P388/DOX-bearing mice were given PSC 833 intraperitoneally 30 min before an intravenous injection of DOX. The levels of DOX were determined by a high-performance liquid chromatography method in leukaemic cells and in normal tissues (heart, lung, liver, small intestine, kidney and spleen). In all tissues, DOX concentrations were significantly increased in mice pretreated with PSC 833. The difference was greatest in P-gp-overexpressing P388/DOX cells, the DOX area under the curve being approximately seven times greater after PSC 833 and DOX than after DOX alone. In P388 cells the difference was approximately 2.5 times, as in the majority of normal tissues. As expected DOX levels in P388 cells were higher than in P388/DOX cells in mice treated with DOX alone, whereas after PSC 833 and DOX the levels of DOX were similar in the two leukaemic lines. In spite of this PSC 833 was unable to reverse the resistance to DOX of P388/DOX leukaemia in vivo, suggesting that mechanisms other than P-gp expression are responsible for resistance.
Full text
PDF
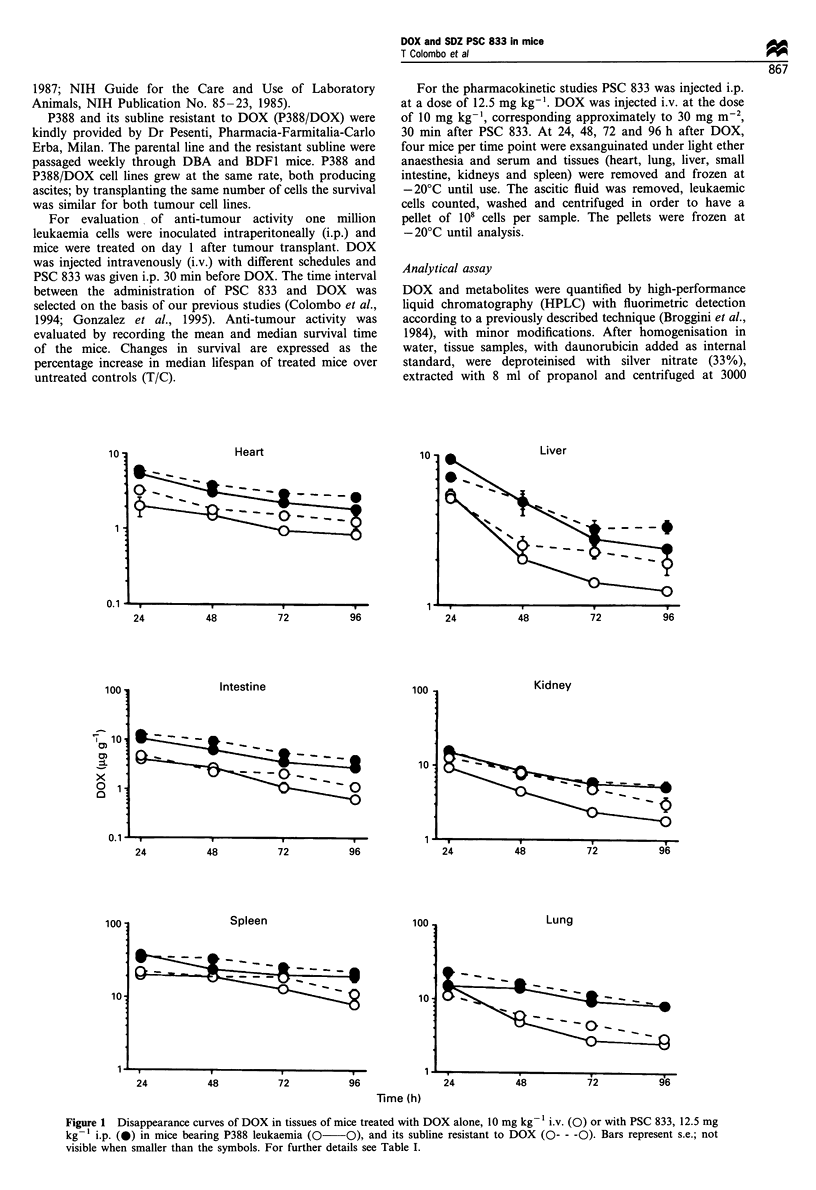
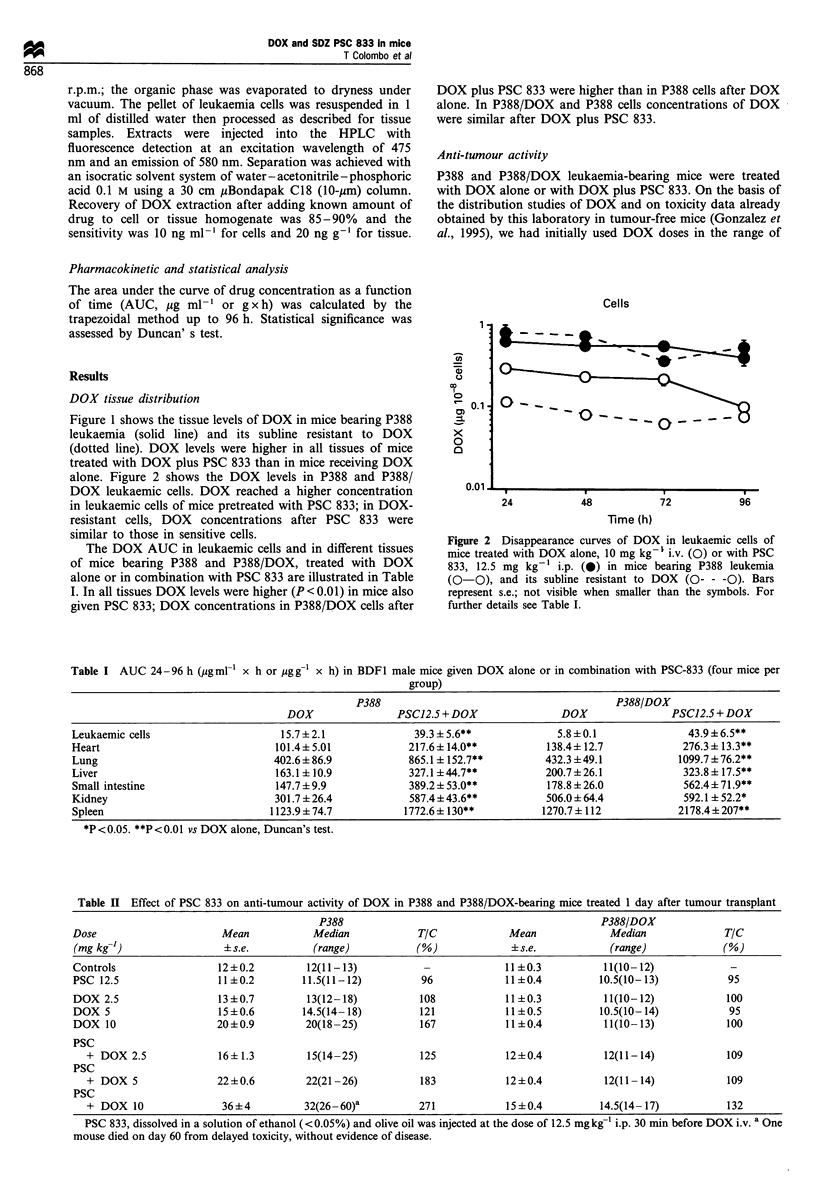
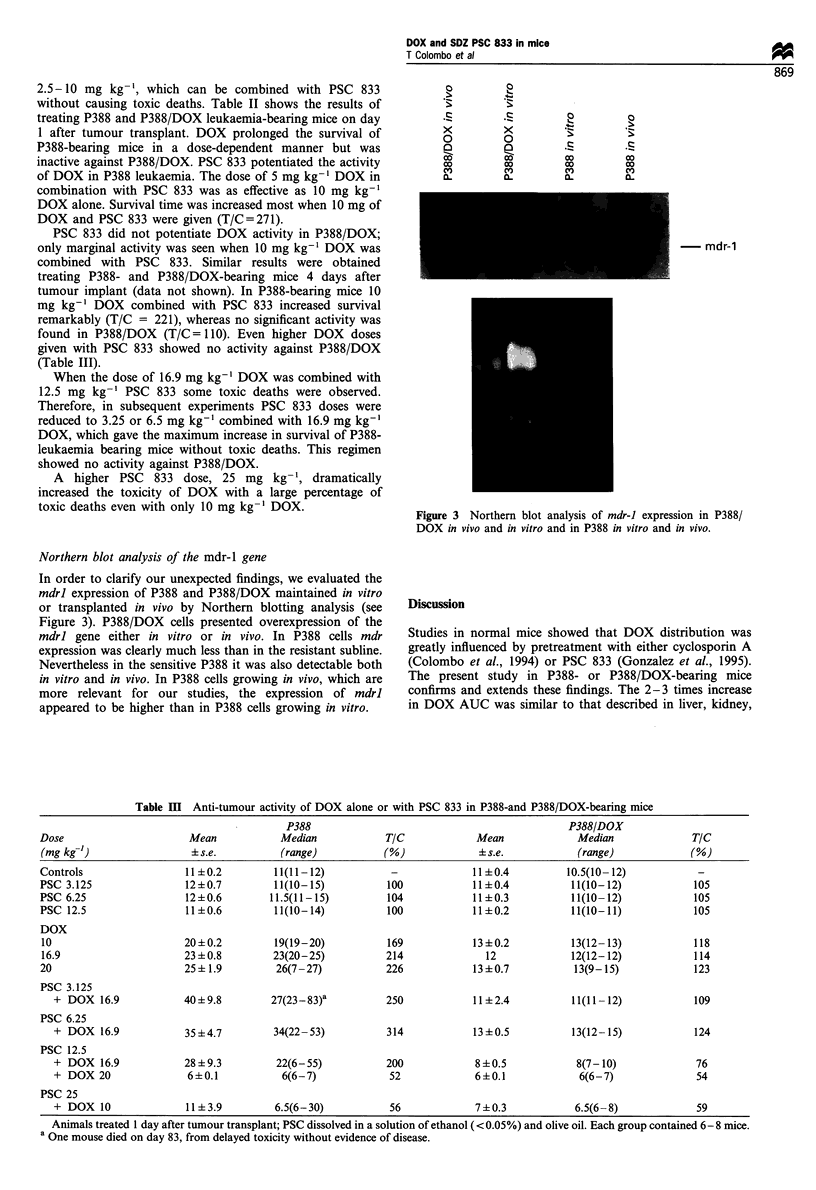


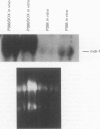
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boesch D., Gavériaux C., Jachez B., Pourtier-Manzanedo A., Bollinger P., Loor F. In vivo circumvention of P-glycoprotein-mediated multidrug resistance of tumor cells with SDZ PSC 833. Cancer Res. 1991 Aug 15;51(16):4226–4233. [PubMed] [Google Scholar]
- Boesch D., Loor F. Extent and persistence of P-glycoprotein inhibition in multidrug-resistant P388 cells after exposure to resistance-modifying agents. Anticancer Drugs. 1994 Apr;5(2):229–238. doi: 10.1097/00001813-199404000-00015. [DOI] [PubMed] [Google Scholar]
- Boesch D., Muller K., Pourtier-Manzanedo A., Loor F. Restoration of daunomycin retention in multidrug-resistant P388 cells by submicromolar concentrations of SDZ PSC 833, a nonimmunosuppressive cyclosporin derivative. Exp Cell Res. 1991 Sep;196(1):26–32. doi: 10.1016/0014-4827(91)90452-z. [DOI] [PubMed] [Google Scholar]
- Broggini M., Italia C., Colombo T., Marmonti L., Donelli M. G. Activity and distribution of iv and oral 4-demethoxydaunorubicin in murine experimental tumors. Cancer Treat Rep. 1984 May;68(5):739–747. [PubMed] [Google Scholar]
- Capranico G., Soranzo C., Zunino F. Single-strand DNA breaks induced by chromophore-modified anthracyclines in P388 leukemia cells. Cancer Res. 1986 Nov;46(11):5499–5503. [PubMed] [Google Scholar]
- Colombo T., Zucchetti M., D'Incalci M. Cyclosporin A markedly changes the distribution of doxorubicin in mice and rats. J Pharmacol Exp Ther. 1994 Apr;269(1):22–27. [PubMed] [Google Scholar]
- Croop J. M., Raymond M., Haber D., Devault A., Arceci R. J., Gros P., Housman D. E. The three mouse multidrug resistance (mdr) genes are expressed in a tissue-specific manner in normal mouse tissues. Mol Cell Biol. 1989 Mar;9(3):1346–1350. doi: 10.1128/mcb.9.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Incalci M. First International Conference on Reversal of Multi-Drug Resistance, St. Gallen, 1-3 September 1994. Ann Oncol. 1994 Dec;5(10):893–894. doi: 10.1093/oxfordjournals.annonc.a058726. [DOI] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Erlichman C., Moore M., Thiessen J. J., Kerr I. G., Walker S., Goodman P., Bjarnason G., DeAngelis C., Bunting P. Phase I pharmacokinetic study of cyclosporin A combined with doxorubicin. Cancer Res. 1993 Oct 15;53(20):4837–4842. [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J. M., Hait W. N. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev. 1990 Sep;42(3):155–199. [PubMed] [Google Scholar]
- Friche E., Jensen P. B., Nissen N. I. Comparison of cyclosporin A and SDZ PSC833 as multidrug-resistance modulators in a daunorubicin-resistant Ehrlich ascites tumor. Cancer Chemother Pharmacol. 1992;30(3):235–237. doi: 10.1007/BF00686321. [DOI] [PubMed] [Google Scholar]
- Gonzalez O., Colombo T., De Fusco M., Imperatori L., Zucchetti M., D'Incalci M. Changes in doxorubicin distribution and toxicity in mice pretreated with the cyclosporin analogue SDZ PSC 833. Cancer Chemother Pharmacol. 1995;36(4):335–340. doi: 10.1007/BF00689051. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M. How cancer cells evade chemotherapy: sixteenth Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. 1993 Feb 15;53(4):747–754. [PubMed] [Google Scholar]
- Gros P., Ben Neriah Y. B., Croop J. M., Housman D. E. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature. 1986 Oct 23;323(6090):728–731. doi: 10.1038/323728a0. [DOI] [PubMed] [Google Scholar]
- Isaacs R. J., Davies S. L., Wells N. J., Harris A. L. Topoisomerases II alpha and beta as therapy targets in breast cancer. Anticancer Drugs. 1995 Apr;6(2):195–211. doi: 10.1097/00001813-199504000-00002. [DOI] [PubMed] [Google Scholar]
- Kaye S. B. P glycoprotein (P-gp) and drug resistance--time for reappraisal? Br J Cancer. 1993 Apr;67(4):641–643. doi: 10.1038/bjc.1993.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. P., Altermatt H. J., Nooter K., Poschmann G., Laissue J. A., Bollinger P., Hiestand P. C. SDZ PSC 833, a non-immunosuppressive cyclosporine: its potency in overcoming P-glycoprotein-mediated multidrug resistance of murine leukemia. Int J Cancer. 1992 Feb 20;50(4):593–597. doi: 10.1002/ijc.2910500418. [DOI] [PubMed] [Google Scholar]
- Radel S., Bankusli I., Mayhew E., Rustum Y. M. The effects of verapamil and a tiapamil analogue, DMDP, on adriamycin-induced cytotoxicity in P388 adriamycin-resistant and -sensitive leukemia in vitro and in vivo. Cancer Chemother Pharmacol. 1988;21(1):25–30. doi: 10.1007/BF00262733. [DOI] [PubMed] [Google Scholar]
- Raderer M., Scheithauer W. Clinical trials of agents that reverse multidrug resistance. A literature review. Cancer. 1993 Dec 15;72(12):3553–3563. doi: 10.1002/1097-0142(19931215)72:12<3553::aid-cncr2820721203>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Rodenburg C. J., Nooter K., Herweijer H., Seynaeve C., Oosterom R., Stoter G., Verweij J. Phase II study of combining vinblastine and cyclosporin-A to circumvent multidrug resistance in renal cell cancer. Ann Oncol. 1991 Apr;2(4):305–306. doi: 10.1093/oxfordjournals.annonc.a057941. [DOI] [PubMed] [Google Scholar]
- Sikic B. I. Modulation of multidrug resistance: at the threshold. J Clin Oncol. 1993 Sep;11(9):1629–1635. doi: 10.1200/JCO.1993.11.9.1629. [DOI] [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruo T., Tomida A. Multidrug resistance. Anticancer Drugs. 1995 Apr;6(2):213–218. doi: 10.1097/00001813-199504000-00003. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R., Bleehen N. M. Resistance modification by PSC-833, a novel non-immunosuppressive cyclosporin [corrected]. Eur J Cancer. 1991;27(12):1639–1642. doi: 10.1016/0277-5379(91)90435-g. [DOI] [PubMed] [Google Scholar]