Abstract
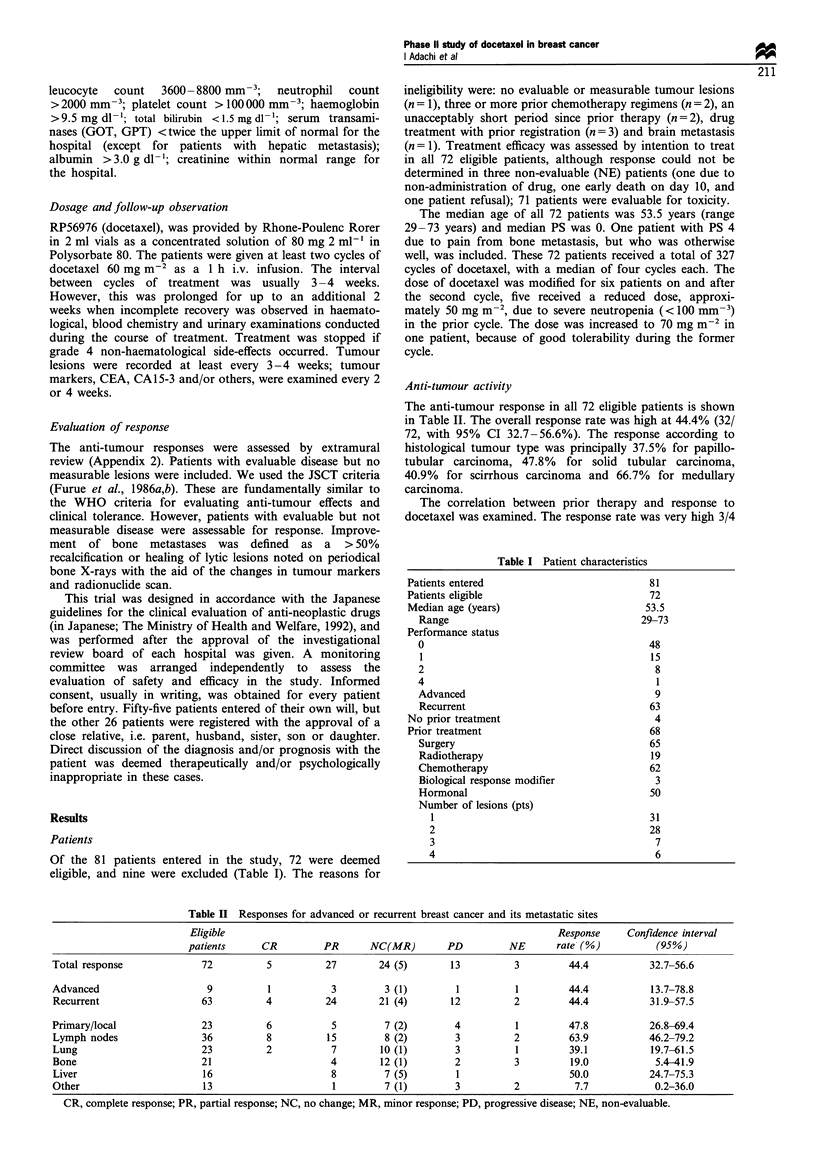
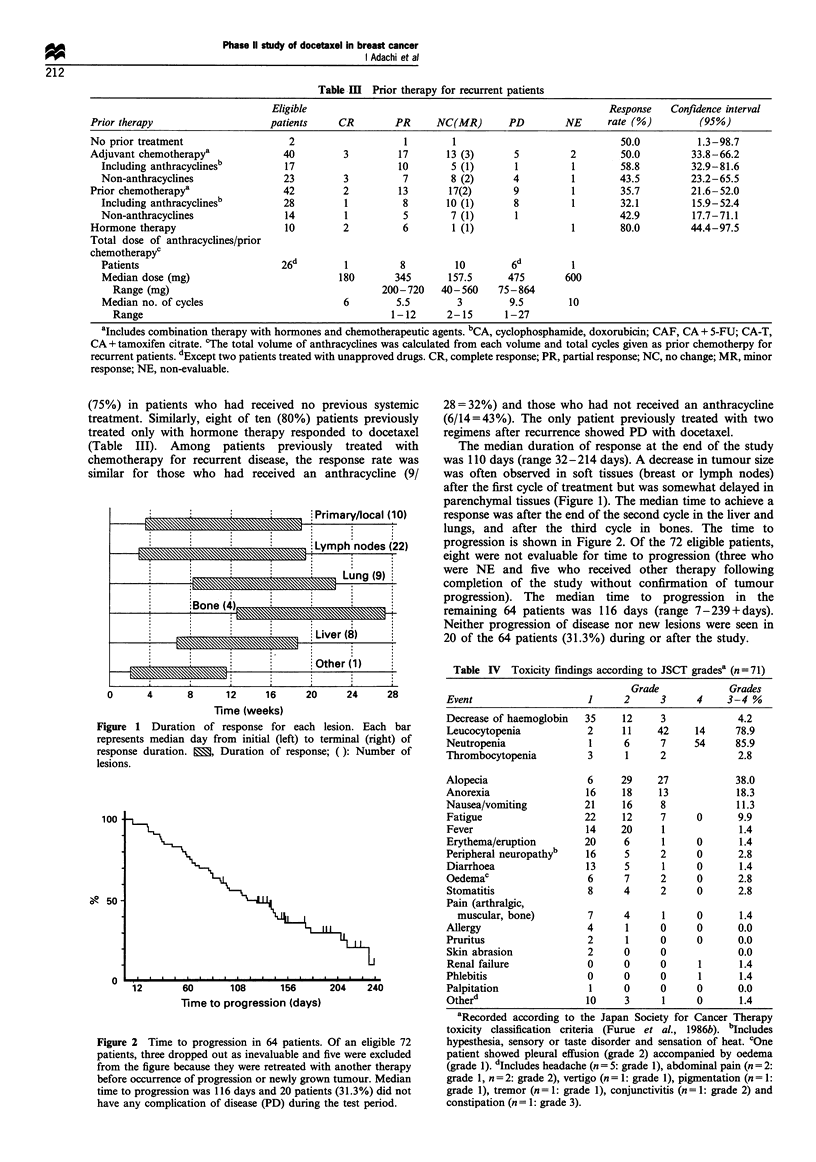
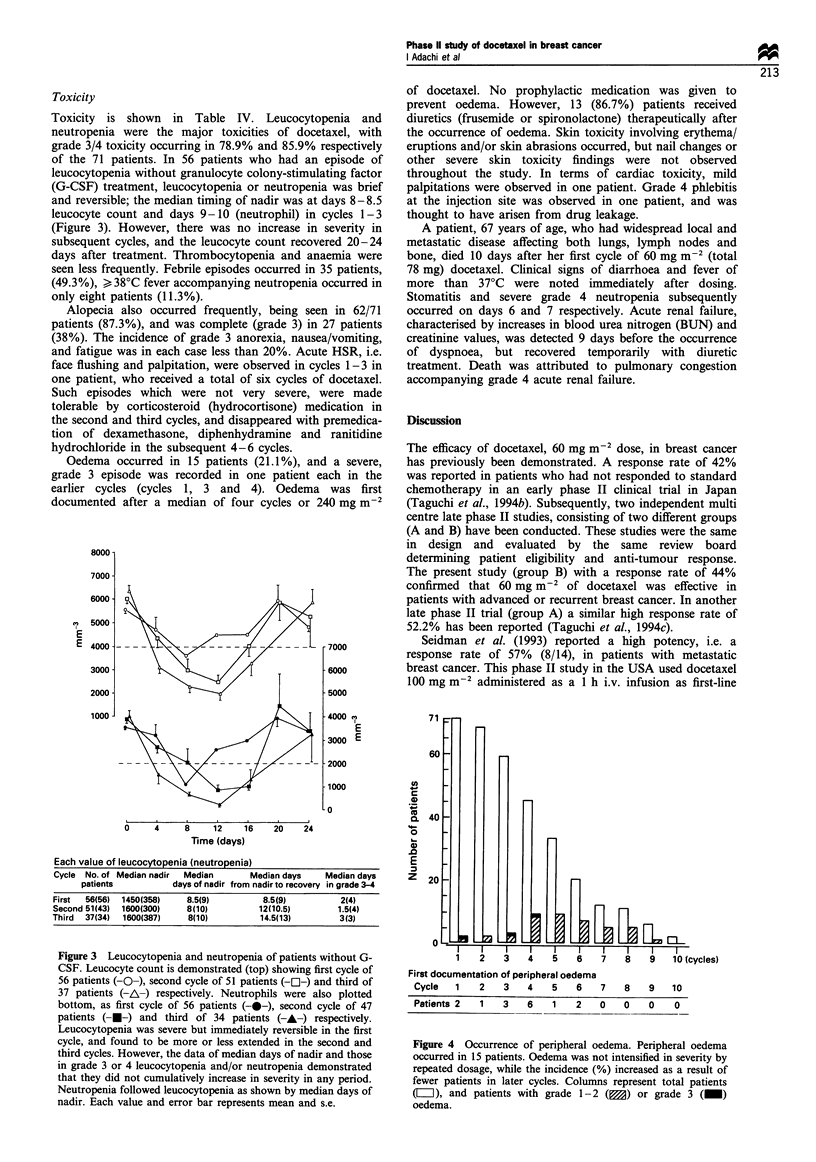
A late phase II clinical trial of RP56976 (docetaxel), derived from Taxus baccata was performed to evaluate anti-tumour activity, time to progression and clinical toxicity in patients with advanced or recurrent breast cancer. The patients, between 15 and 80 years old with performance status (PS) of 0-2, received at least two cycles of docetaxel 60 mg m-2 intravenously at 3-4 week intervals. Of the 81 patients enrolled, the 72 eligible for the study were given a total of 327 cycles, with a median of four cycles each. Five patients obtained a complete response (CR) and 27 a partial response (PR); the response rate (RR) was 44.4% (95% confidence interval 32.7-56.6%). A relatively high RR of 9/28 (32.1%) was observed in patients who had received prior chemotherapy involving anthracyclines. The dose-limiting toxicity was grade 3-4 leucocytopenia or neutropenia, found in 78.9% and 85.9% patients respectively. Other severe (grade > 3) toxicities included alopecia (38%), anorexia (18.3%), nausea/vomiting (11.3%), and fatigue (9.9%). Hypersensitivity reactions, oedema and skin toxicity were not severe and were reversible. One therapy-related death occurred 10 days after the initial dose was given. These findings indicate that docetaxel has potent activity against metastatic breast cancer, and that the dose of 60 mg m-2 is safe.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissery M. C., Guénard D., Guéritte-Voegelein F., Lavelle F. Experimental antitumor activity of taxotere (RP 56976, NSC 628503), a taxol analogue. Cancer Res. 1991 Sep 15;51(18):4845–4852. [PubMed] [Google Scholar]
- Burris H., Irvin R., Kuhn J., Kalter S., Smith L., Shaffer D., Fields S., Weiss G., Eckardt J., Rodriguez G. Phase I clinical trial of taxotere administered as either a 2-hour or 6-hour intravenous infusion. J Clin Oncol. 1993 May;11(5):950–958. doi: 10.1200/JCO.1993.11.5.950. [DOI] [PubMed] [Google Scholar]
- Gregory W. M., Smith P., Richards M. A., Twelves C. J., Knight R. K., Rubens R. D. Chemotherapy of advanced breast cancer: outcome and prognostic factors. Br J Cancer. 1993 Nov;68(5):988–995. doi: 10.1038/bjc.1993.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes F. A., Walters R. S., Theriault R. L., Forman A. D., Newton L. K., Raber M. N., Buzdar A. U., Frye D. K., Hortobagyi G. N. Phase II trial of taxol, an active drug in the treatment of metastatic breast cancer. J Natl Cancer Inst. 1991 Dec 18;83(24):1797–1805. doi: 10.1093/jnci/83.24.1797-a. [DOI] [PubMed] [Google Scholar]
- Kelland L. R., Abel G. Comparative in vitro cytotoxicity of taxol and Taxotere against cisplatin-sensitive and -resistant human ovarian carcinoma cell lines. Cancer Chemother Pharmacol. 1992;30(6):444–450. doi: 10.1007/BF00685595. [DOI] [PubMed] [Google Scholar]
- Kubo K., Abe O., Izuo M., Enomoto K., Koyama H., Sakai K., Terasawa T., Tominaga T., Nomura Y. [Clinical evaluation of adriamycin for advanced breast cancer (3)--a joint study by 26 institutes on the CAF and CMcF treatment]. Gan To Kagaku Ryoho. 1983 Dec;10(12):2523–2531. [PubMed] [Google Scholar]
- Pazdur R., Kudelka A. P., Kavanagh J. J., Cohen P. R., Raber M. N. The taxoids: paclitaxel (Taxol) and docetaxel (Taxotere). Cancer Treat Rev. 1993 Oct;19(4):351–386. doi: 10.1016/0305-7372(93)90010-o. [DOI] [PubMed] [Google Scholar]
- Ringel I., Horwitz S. B. Studies with RP 56976 (taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst. 1991 Feb 20;83(4):288–291. doi: 10.1093/jnci/83.4.288. [DOI] [PubMed] [Google Scholar]
- Weiss R. B., Donehower R. C., Wiernik P. H., Ohnuma T., Gralla R. J., Trump D. L., Baker J. R., Jr, Van Echo D. A., Von Hoff D. D., Leyland-Jones B. Hypersensitivity reactions from taxol. J Clin Oncol. 1990 Jul;8(7):1263–1268. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]


