Abstract
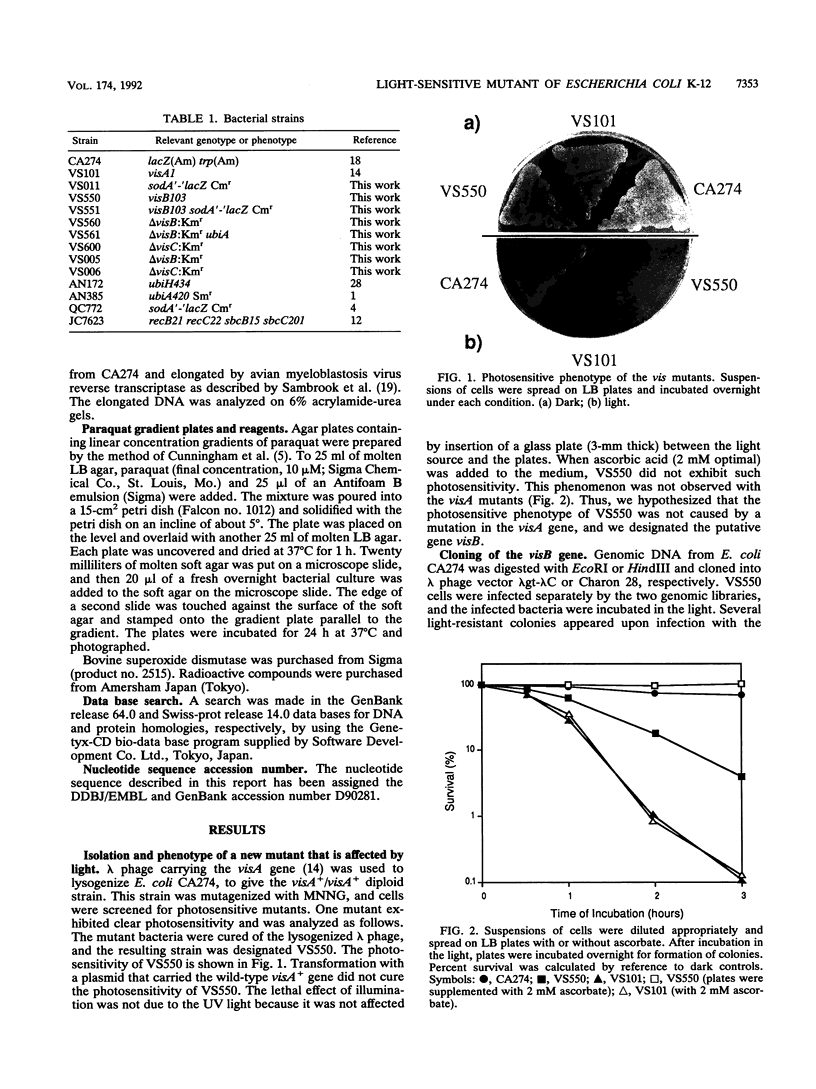
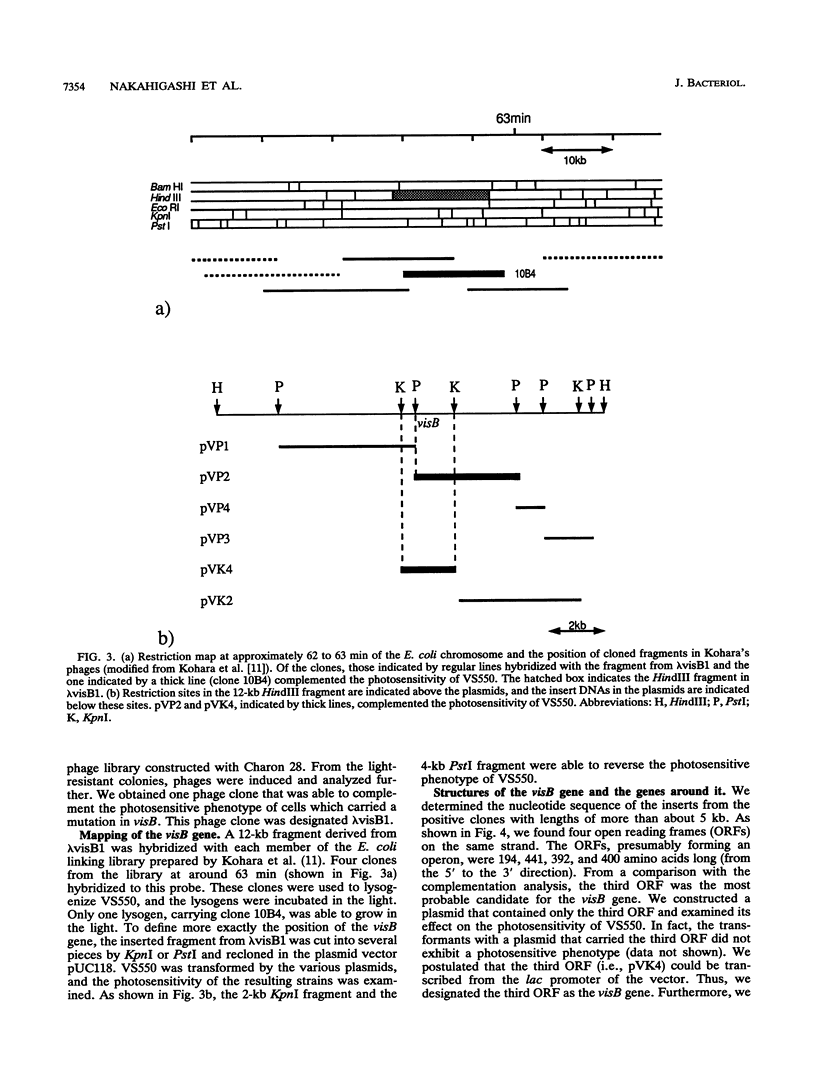
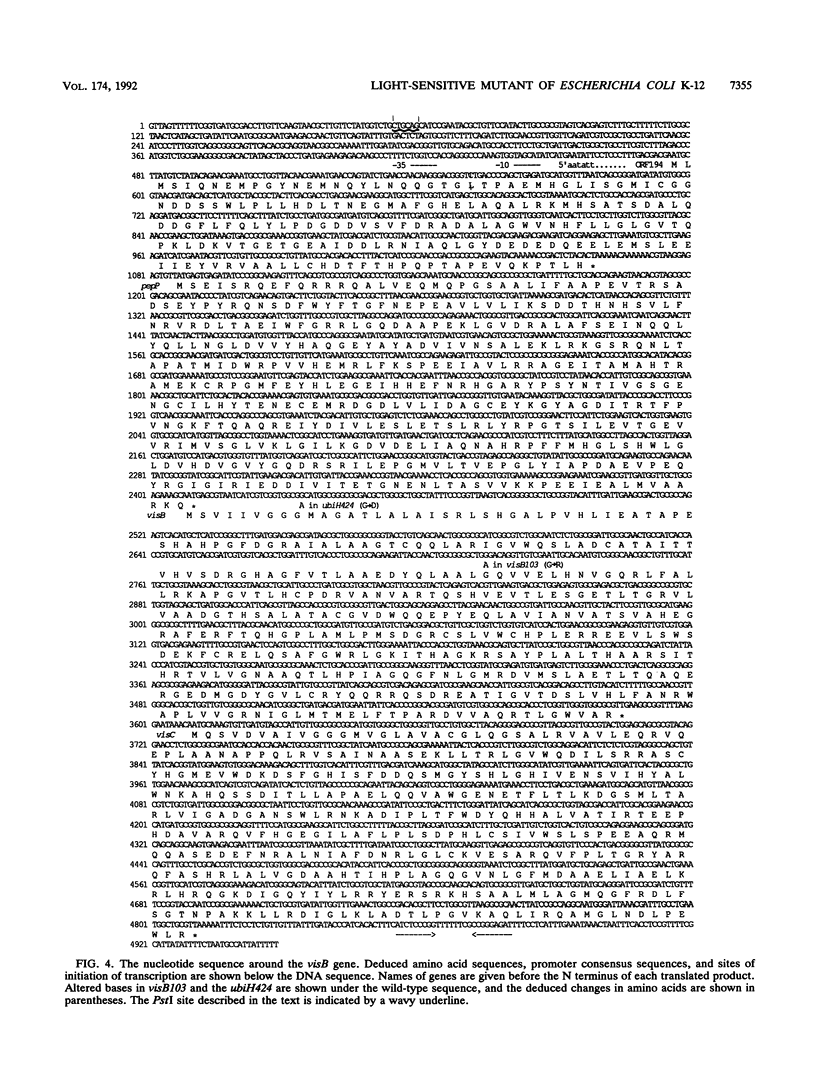
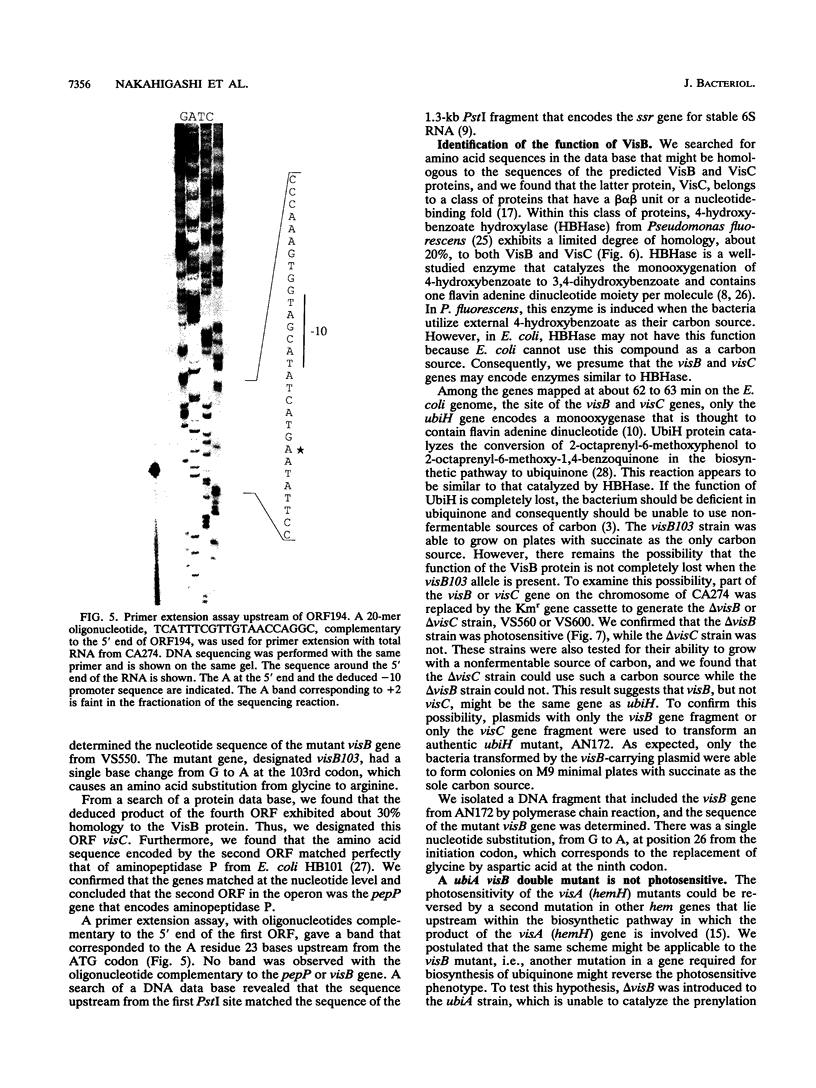
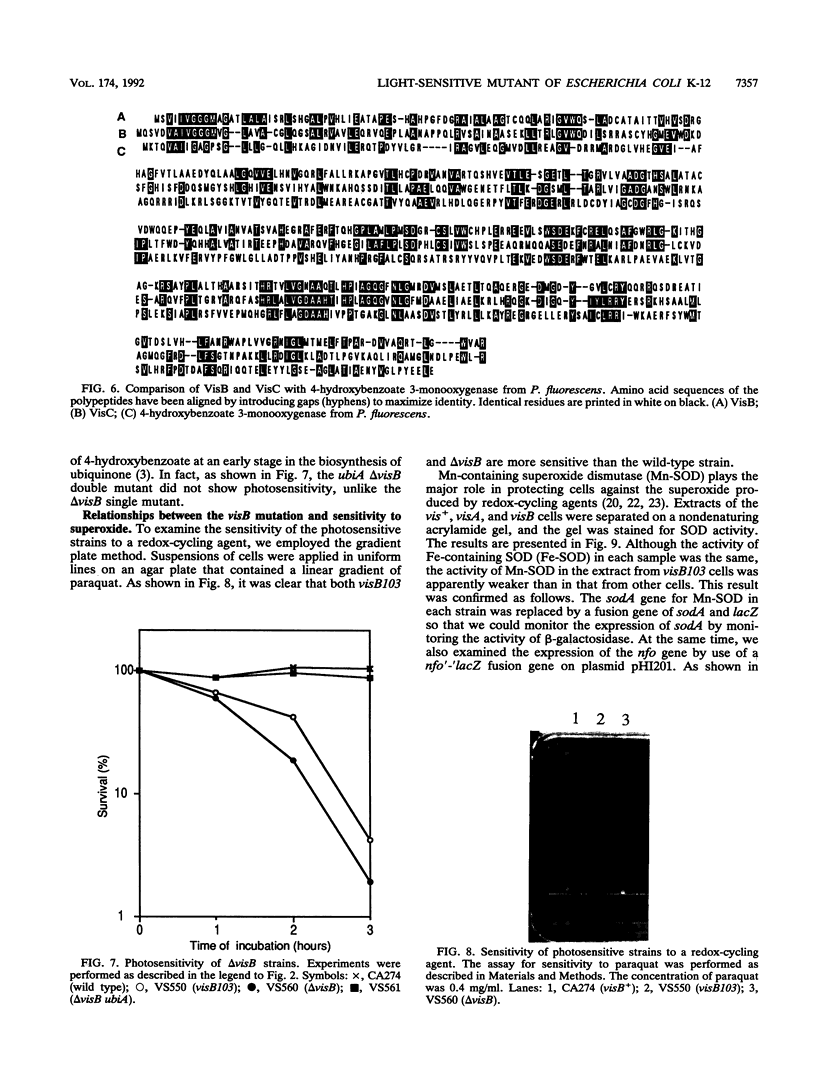
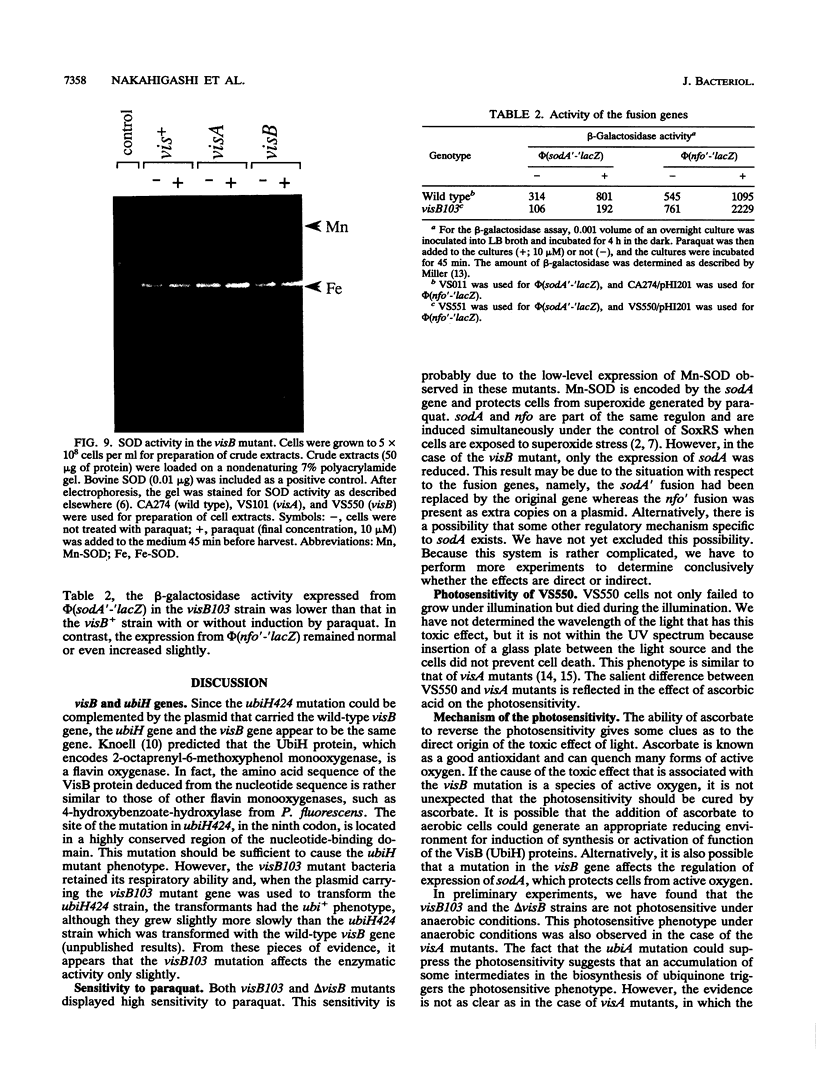
Cells with a novel mutation that is lethal when the cells are exposed to visible light were isolated from Escherichia coli K-12. The mutation was mapped at 63 min on the linkage map of the E. coli chromosome, and the gene, designated visB, was cloned and sequenced. From its map position and the evidence that the gene product VisB exhibits homology with flavin monooxygenase of Pseudomonas fluorescens, the visB gene was deduced to be identical to the ubiH gene, which is a gene required for the biosynthesis of ubiquinone and is thought to be similar to the gene for flavin monooxygenase. The photosensitive phenotype appears to be due to the accumulation of the substrate for the reaction catalyzed by the visB (ubiH) gene product because other mutations that block earlier steps in the biosynthesis of ubiquinone can reverse the photosensitivity. The accumulated intermediates may produce active species of oxygen in the mutant bacteria upon illumination by visible light, and these active oxygen species may cause the death of the cells by a mechanism similar to that associated with mutations in visA (hemH).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander K., Young I. G. Alternative hydroxylases for the aerobic and anaerobic biosynthesis of ubiquinone in Escherichia coli. Biochemistry. 1978 Oct 31;17(22):4750–4755. doi: 10.1021/bi00615a024. [DOI] [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham R. P., Saporito S. M., Spitzer S. G., Weiss B. Endonuclease IV (nfo) mutant of Escherichia coli. J Bacteriol. 1986 Dec;168(3):1120–1127. doi: 10.1128/jb.168.3.1120-1127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elroy-Stein O., Bernstein Y., Groner Y. Overproduction of human Cu/Zn-superoxide dismutase in transfected cells: extenuation of paraquat-mediated cytotoxicity and enhancement of lipid peroxidation. EMBO J. 1986 Mar;5(3):615–622. doi: 10.1002/j.1460-2075.1986.tb04255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. T., Monach P., Chou J. H., Josephy P. D., Demple B. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6181–6185. doi: 10.1073/pnas.87.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofsteenge J., Vereijken J. M., Weijer W. J., Beintema J. J., Wierenga R. K., Drenth J. Primary and tertiary structure studies of p-hydroxybenzoate hydroxylase from Pseudomonas fluorescens. Isolation and alignment of the CNBr peptides; interactions of the protein with flavin adenine dinucleotide. Eur J Biochem. 1980 Dec;113(1):141–150. [PubMed] [Google Scholar]
- Hsu L. M., Zagorski J., Wang Z., Fournier M. J. Escherichia coli 6S RNA gene is part of a dual-function transcription unit. J Bacteriol. 1985 Mar;161(3):1162–1170. doi: 10.1128/jb.161.3.1162-1170.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Templin A., Clark A. J. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc Natl Acad Sci U S A. 1971 Apr;68(4):824–827. doi: 10.1073/pnas.68.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K., Nakahigashi K., Nishimura K., Inokuchi H. Isolation and characterization of visible light-sensitive mutants of Escherichia coli K12. J Mol Biol. 1991 Jun 5;219(3):393–398. doi: 10.1016/0022-2836(91)90180-e. [DOI] [PubMed] [Google Scholar]
- Nakahigashi K., Nishimura K., Miyamoto K., Inokuchi H. Photosensitivity of a protoporphyrin-accumulating, light-sensitive mutant (visA) of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10520–10524. doi: 10.1073/pnas.88.23.10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm D. L., Horness D., Kucera J., Blattner F. R. Construction of coliphage lambda Charon vectors with BamHI cloning sites. Gene. 1980 Dec;12(3-4):301–309. doi: 10.1016/0378-1119(80)90113-4. [DOI] [PubMed] [Google Scholar]
- Russell R. L., Abelson J. N., Landy A., Gefter M. L., Brenner S., Smith J. D. Duplicate genes for tyrosine transfer RNA in Escherichia coli. J Mol Biol. 1970 Jan 14;47(1):1–13. doi: 10.1016/0022-2836(70)90397-9. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Avila H. Structure and gene expression of the E. coli Mn-superoxide dismutase gene. Nucleic Acids Res. 1986 Jun 11;14(11):4577–4589. doi: 10.1093/nar/14.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Cameron J. R., Davis R. W. Viable molecular hybrids of bacteriophage lambda and eukaryotic DNA. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4579–4583. doi: 10.1073/pnas.71.11.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati D. Cloning and mapping of the manganese superoxide dismutase gene (sodA) of Escherichia coli K-12. J Bacteriol. 1983 Sep;155(3):1078–1087. doi: 10.1128/jb.155.3.1078-1087.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati D. Transcriptional and posttranscriptional regulation of manganese superoxide dismutase biosynthesis in Escherichia coli, studied with operon and protein fusions. J Bacteriol. 1988 Jun;170(6):2511–2520. doi: 10.1128/jb.170.6.2511-2520.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Weijer W. J., Hofsteenge J., Vereijken J. M., Jekel P. A., Beintema J. J. Primary structure of p-hydroxybenzoate hydroxylase from Pseudomonas fluorescens. Biochim Biophys Acta. 1982 Jun 4;704(2):385–388. doi: 10.1016/0167-4838(82)90170-4. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., de Jong R. J., Kalk K. H., Hol W. G., Drenth J. Crystal structure of p-hydroxybenzoate hydroxylase. J Mol Biol. 1979 Jun 15;131(1):55–73. doi: 10.1016/0022-2836(79)90301-2. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Tone H., Honda T., Osatomi K., Kobayashi R., Tsuru D. Sequencing and high expression of aminopeptidase P gene from Escherichia coli HB101. J Biochem. 1989 Mar;105(3):412–416. doi: 10.1093/oxfordjournals.jbchem.a122678. [DOI] [PubMed] [Google Scholar]
- Young I. G., Stroobant P., Macdonald C. G., Gibson F. Pathway for ubiquinone biosynthesis in Escherichia coli K-12: gene-enzyme relationships and intermediates. J Bacteriol. 1973 Apr;114(1):42–52. doi: 10.1128/jb.114.1.42-52.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]