Abstract
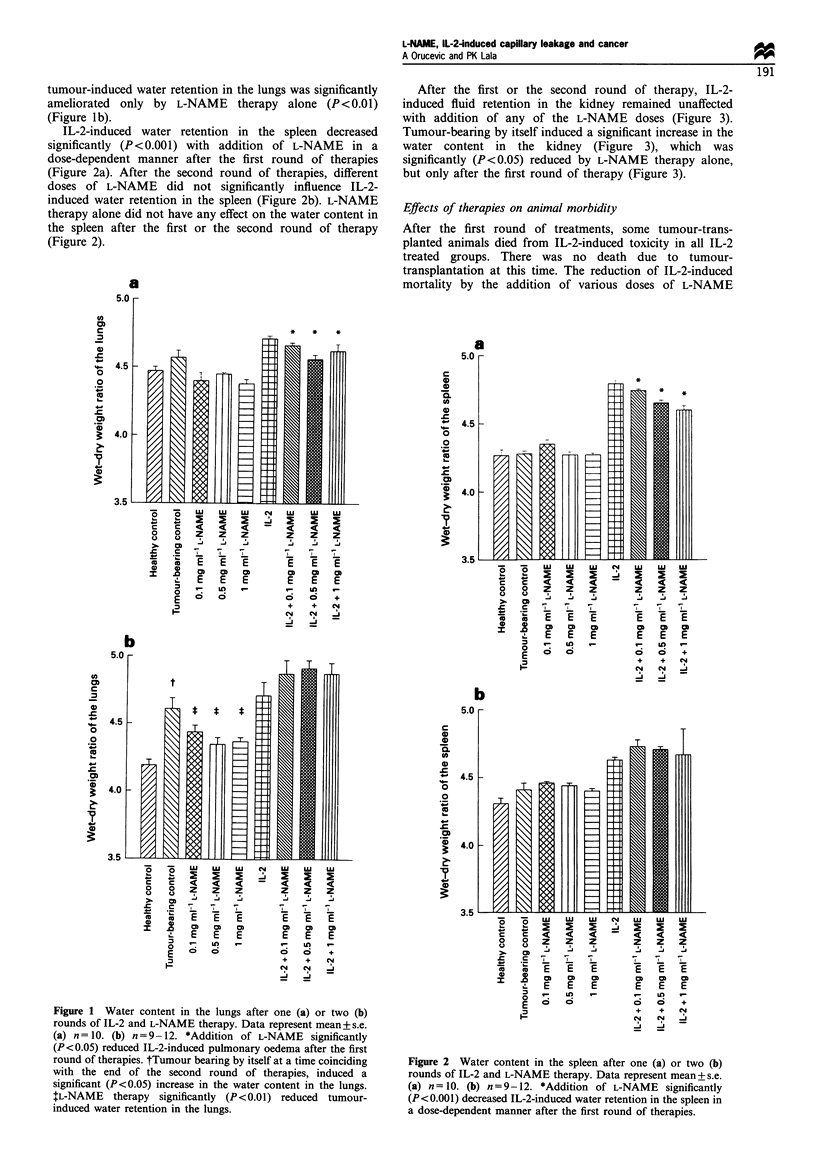
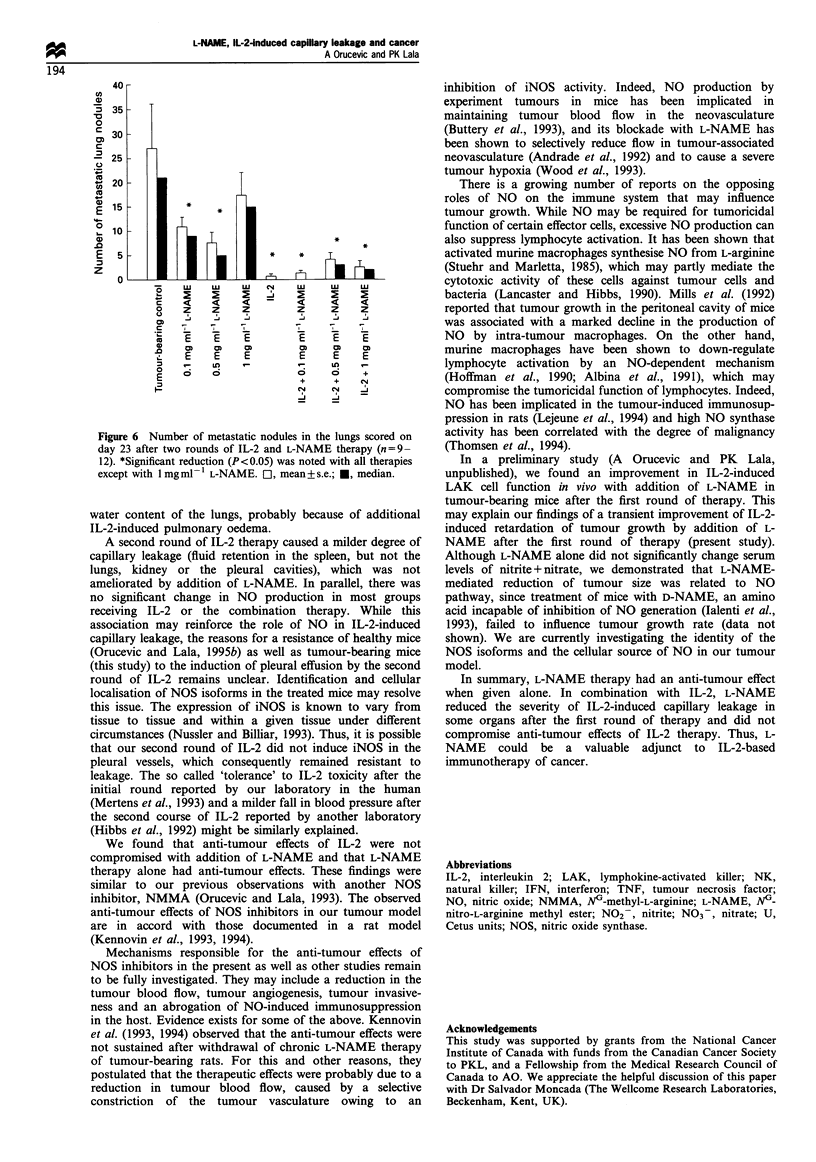
We tested whether NG-nitro-L-arginine methyl ester (L-NAME), an inhibitor of nitric oxide (NO) synthesis, can prevent interleukin 2 (IL-2)-induced capillary leakage in tumour-bearing mice without compromising the therapeutic benefits of IL-2. C3H/HeJ female mice transplanted s.c. with 2.5 x 10(5) C3-L5 mammary carcinoma cells were treated with: nothing, IL-2 (ten injections of 15,000 Cetus units i.p. every 8 h), L-NAME (0.1, 0.5, or 1 mg ml-1 drinking water), IL-2 + L-NAME (0.1 or 0.5 or 1 mg ml-1 drinking water). Therapies were given in one round (IL-2, days 10-13; L-NAME, days 9-13) or in two rounds (IL-2, days 10-13 and 20-23; L-NAME, days 9-13 and days 19-23) after tumour transplantation. Capillary leakage was measured from the water contents of the pleural cavities, lungs, spleen and kidneys. Effects of the therapies on the primary tumour size and the number of spontaneous lung metastases were also recorded. NO production was measured as the nitrite + nitrate levels in the serum and in the pleural effusion. After the first round of therapies, addition of L-NAME significantly reduced IL-2-induced pulmonary oedema and water retention in the spleen in a dose-dependent manner. It also significantly reduced the IL-2-induced rise in NO levels in the serum and pleural fluid, but did not affect IL-2-induced pleural effusion or water retention in the kidney. At later stages of tumour growth (day 23), tumours themselves induced significant fluid retention in the lungs and the kidney, which was not aggravated further with the second round of IL-2 therapy. At this time, L-NAME therapy alone ameliorated tumour-induced pulmonary oedema. During both rounds of therapy different doses of L-NAME alone caused a reduction of primary tumour growth as well as spontaneous lung metastases, which improved further with the addition of IL-2. The combination therapy was at least as effective as IL-2 therapy. In summary, L-NAME had anti-tumour effects in vivo, reduced the severity of IL-2-induced capillary leakage in some organs and did not compromise anti-tumour efficacy of IL-2 therapy. Thus, L-NAME could be a valuable adjunct to IL-2-based cancer therapy.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albina J. E., Abate J. A., Henry W. L., Jr Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation. Role of IFN-gamma in the induction of the nitric oxide-synthesizing pathway. J Immunol. 1991 Jul 1;147(1):144–148. [PubMed] [Google Scholar]
- Amador J. F., Vazquez A. M., Cabrera L., Barral A. M., Gendelman R., Jondal M. Toxic effects of interleukin-2-activated lymphocytes on vascular endothelial cells. Nat Immun Cell Growth Regul. 1991;10(4):207–215. [PubMed] [Google Scholar]
- Andrade S. P., Hart I. R., Piper P. J. Inhibitors of nitric oxide synthase selectively reduce flow in tumor-associated neovasculature. Br J Pharmacol. 1992 Dec;107(4):1092–1095. doi: 10.1111/j.1476-5381.1992.tb13412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson F. R., Libby P., Brandon E. P., Janicka M. W., Mier J. W. IL-2 rapidly induces natural killer cell adhesion to human endothelial cells. A potential mechanism for endothelial injury. J Immunol. 1988 Jul 1;141(1):158–163. [PubMed] [Google Scholar]
- Baguley B. C., Calveley S. B., Crowe K. K., Fray L. M., O'Rourke S. A., Smith G. P. Comparison of the effects of flavone acetic acid, fostriecin, homoharringtonine and tumour necrosis factor alpha on colon 38 tumours in mice. Eur J Cancer Clin Oncol. 1989 Feb;25(2):263–269. doi: 10.1016/0277-5379(89)90018-7. [DOI] [PubMed] [Google Scholar]
- Brodt P., Parhar R., Sankar P., Lala P. K. Studies on clonal heterogeneity in two spontaneously metastasizing mammary carcinomas of recent origin. Int J Cancer. 1985 Feb 15;35(2):265–273. doi: 10.1002/ijc.2910350220. [DOI] [PubMed] [Google Scholar]
- Buttery L. D., Springall D. R., Andrade S. P., Riveros-Moreno V., Hart I., Piper P. J., Polak J. M. Induction of nitric oxide synthase in the neo-vasculature of experimental tumours in mice. J Pathol. 1993 Dec;171(4):311–319. doi: 10.1002/path.1711710412. [DOI] [PubMed] [Google Scholar]
- Dutcher J. P., Creekmore S., Weiss G. R., Margolin K., Markowitz A. B., Roper M., Parkinson D., Ciobanu N., Fisher R. I., Boldt D. H. A phase II study of interleukin-2 and lymphokine-activated killer cells in patients with metastatic malignant melanoma. J Clin Oncol. 1989 Apr;7(4):477–485. doi: 10.1200/JCO.1989.7.4.477. [DOI] [PubMed] [Google Scholar]
- Dwyer M. A., Bredt D. S., Snyder S. H. Nitric oxide synthase: irreversible inhibition by L-NG-nitroarginine in brain in vitro and in vivo. Biochem Biophys Res Commun. 1991 May 15;176(3):1136–1141. doi: 10.1016/0006-291x(91)90403-t. [DOI] [PubMed] [Google Scholar]
- Ettinghausen S. E., Puri R. K., Rosenberg S. A. Increased vascular permeability in organs mediated by the systemic administration of lymphokine-activated killer cells and recombinant interleukin-2 in mice. J Natl Cancer Inst. 1988 Apr 6;80(3):177–188. doi: 10.1093/jnci/80.3.177. [DOI] [PubMed] [Google Scholar]
- Faggioni R., Allavena P., Cantoni L., Carelli M., Demitri M. T., Delgado R., Gatti S., Gnocchi P., Isetta A. M., Paganin C. Mechanisms of interleukin-2-induced hydrothoraxy in mice: protective effect of endotoxin tolerance and dexamethasone and possible role of reactive oxygen intermediates. J Immunother Emphasis Tumor Immunol. 1994 Apr;15(3):194–201. [PubMed] [Google Scholar]
- Fisher R. I., Coltman C. A., Jr, Doroshow J. H., Rayner A. A., Hawkins M. J., Mier J. W., Wiernik P., McMannis J. D., Weiss G. R., Margolin K. A. Metastatic renal cancer treated with interleukin-2 and lymphokine-activated killer cells. A phase II clinical trial. Ann Intern Med. 1988 Apr;108(4):518–523. doi: 10.7326/0003-4819-108-4-518. [DOI] [PubMed] [Google Scholar]
- Furfine E. S., Harmon M. F., Paith J. E., Garvey E. P. Selective inhibition of constitutive nitric oxide synthase by L-NG-nitroarginine. Biochemistry. 1993 Aug 24;32(33):8512–8517. doi: 10.1021/bi00084a017. [DOI] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Westenfelder C., Taintor R., Vavrin Z., Kablitz C., Baranowski R. L., Ward J. H., Menlove R. L., McMurry M. P., Kushner J. P. Evidence for cytokine-inducible nitric oxide synthesis from L-arginine in patients receiving interleukin-2 therapy. J Clin Invest. 1992 Mar;89(3):867–877. doi: 10.1172/JCI115666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R. A., Langrehr J. M., Billiar T. R., Curran R. D., Simmons R. L. Alloantigen-induced activation of rat splenocytes is regulated by the oxidative metabolism of L-arginine. J Immunol. 1990 Oct 1;145(7):2220–2226. [PubMed] [Google Scholar]
- Ialenti A., Moncada S., Di Rosa M. Modulation of adjuvant arthritis by endogenous nitric oxide. Br J Pharmacol. 1993 Oct;110(2):701–706. doi: 10.1111/j.1476-5381.1993.tb13868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahaleh M. B., Smith E. A., Soma Y., LeRoy E. C. Effect of lymphotoxin and tumor necrosis factor on endothelial and connective tissue cell growth and function. Clin Immunol Immunopathol. 1988 Nov;49(2):261–272. doi: 10.1016/0090-1229(88)90116-x. [DOI] [PubMed] [Google Scholar]
- Lala P. K., Parhar R. S. Eradication of spontaneous and experimental adenocarcinoma metastases with chronic indomethacin and intermittent IL-2 therapy. Int J Cancer. 1993 Jun 19;54(4):677–684. doi: 10.1002/ijc.2910540425. [DOI] [PubMed] [Google Scholar]
- Lala P. K., Parhar R. S., Singh P. Indomethacin therapy abrogates the prostaglandin-mediated suppression of natural killer activity in tumor-bearing mice and prevents tumor metastasis. Cell Immunol. 1986 Apr 15;99(1):108–118. doi: 10.1016/0008-8749(86)90220-0. [DOI] [PubMed] [Google Scholar]
- Lancaster J. R., Jr, Hibbs J. B., Jr EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1223–1227. doi: 10.1073/pnas.87.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune P., Lagadec P., Onier N., Pinard D., Ohshima H., Jeannin J. F. Nitric oxide involvement in tumor-induced immunosuppression. J Immunol. 1994 May 15;152(10):5077–5083. [PubMed] [Google Scholar]
- Margolin K. A., Rayner A. A., Hawkins M. J., Atkins M. B., Dutcher J. P., Fisher R. I., Weiss G. R., Doroshow J. H., Jaffe H. S., Roper M. Interleukin-2 and lymphokine-activated killer cell therapy of solid tumors: analysis of toxicity and management guidelines. J Clin Oncol. 1989 Apr;7(4):486–498. doi: 10.1200/JCO.1989.7.4.486. [DOI] [PubMed] [Google Scholar]
- Mertens W. C., Bramwell V. H., Banerjee D., Gwadry-Sridhar F., al-Mutter N., Parhar R. S., Lala P. K. Sustained oral indomethacin and ranitidine with intermittent continuous infusion interleukin-2 in advanced renal cell carcinoma. Cancer Biother. 1993 Fall;8(3):229–233. doi: 10.1089/cbr.1993.8.229. [DOI] [PubMed] [Google Scholar]
- Mills C. D., Shearer J., Evans R., Caldwell M. D. Macrophage arginine metabolism and the inhibition or stimulation of cancer. J Immunol. 1992 Oct 15;149(8):2709–2714. [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Montesano R., Orci L., Vassalli P. Human endothelial cell cultures: phenotypic modulation by leukocyte interleukins. J Cell Physiol. 1985 Mar;122(3):424–434. doi: 10.1002/jcp.1041220313. [DOI] [PubMed] [Google Scholar]
- Nussler A. K., Billiar T. R. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993 Aug;54(2):171–178. [PubMed] [Google Scholar]
- Ochoa J. B., Curti B., Peitzman A. B., Simmons R. L., Billiar T. R., Hoffman R., Rault R., Longo D. L., Urba W. J., Ochoa A. C. Increased circulating nitrogen oxides after human tumor immunotherapy: correlation with toxic hemodynamic changes. J Natl Cancer Inst. 1992 Jun 3;84(11):864–867. doi: 10.1093/jnci/84.11.864. [DOI] [PubMed] [Google Scholar]
- Parkinson D. R., Fisher R. I., Rayner A. A., Paietta E., Margolin K. A., Weiss G. R., Mier J. W., Sznol M., Gaynor E. R., Bar M. H. Therapy of renal cell carcinoma with interleukin-2 and lymphokine-activated killer cells: phase II experience with a hybrid bolus and continuous infusion interleukin-2 regimen. J Clin Oncol. 1990 Oct;8(10):1630–1636. doi: 10.1200/JCO.1990.8.10.1630. [DOI] [PubMed] [Google Scholar]
- Puri R. K., Travis W. D., Rosenberg S. A. Decrease in interleukin 2-induced vascular leakage in the lungs of mice by administration of recombinant interleukin 1 alpha in vivo. Cancer Res. 1989 Feb 15;49(4):969–976. [PubMed] [Google Scholar]
- Rosenberg S. A. Clinical immunotherapy studies in the Surgery Branch of the U.S. National Cancer Institute: brief review. Cancer Treat Rev. 1989 Jun;16 (Suppl A):115–121. doi: 10.1016/0305-7372(89)90031-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Chang A. E., Avis F. P., Leitman S., Linehan W. M., Robertson C. N., Lee R. E., Rubin J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987 Apr 9;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Mulé J. J., Spiess P. J., Reichert C. M., Schwarz S. L. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med. 1985 May 1;161(5):1169–1188. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein M., Ettinghausen S. E., Rosenberg S. A. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol. 1986 Sep 1;137(5):1735–1742. [PubMed] [Google Scholar]
- Saarloos M. N., Khoo N. K., Lala P. K. Effects of cancer immunotherapy with indomethacin and interleukin-2 on murine hemopoietic stem cells. Cancer Res. 1992 Dec 1;52(23):6452–6462. [PubMed] [Google Scholar]
- Siegel J. P., Puri R. K. Interleukin-2 toxicity. J Clin Oncol. 1991 Apr;9(4):694–704. doi: 10.1200/JCO.1991.9.4.694. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen L. L., Lawton F. G., Knowles R. G., Beesley J. E., Riveros-Moreno V., Moncada S. Nitric oxide synthase activity in human gynecological cancer. Cancer Res. 1994 Mar 1;54(5):1352–1354. [PubMed] [Google Scholar]
- Welbourn R., Goldman G., Kobzik L., Valeri C. R., Hechtman H. B., Shepro D. Attenuation of IL-2-induced multisystem organ edema by phalloidin and antamanide. J Appl Physiol (1985) 1991 Mar;70(3):1364–1368. doi: 10.1152/jappl.1991.70.3.1364. [DOI] [PubMed] [Google Scholar]
- Wood P. J., Stratford I. J., Adams G. E., Szabo C., Thiemermann C., Vane J. R. Modification of energy metabolism and radiation response of a murine tumour by changes in nitric oxide availability. Biochem Biophys Res Commun. 1993 Apr 30;192(2):505–510. doi: 10.1006/bbrc.1993.1444. [DOI] [PubMed] [Google Scholar]


