Abstract
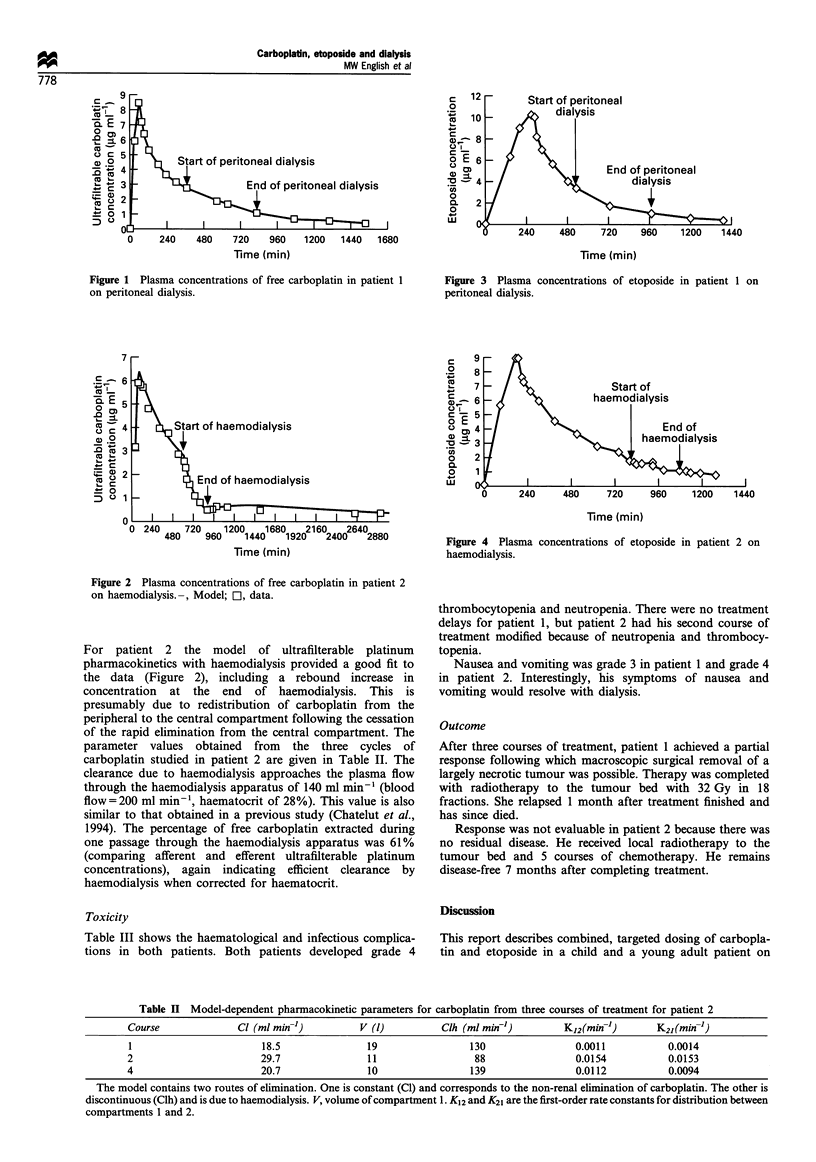
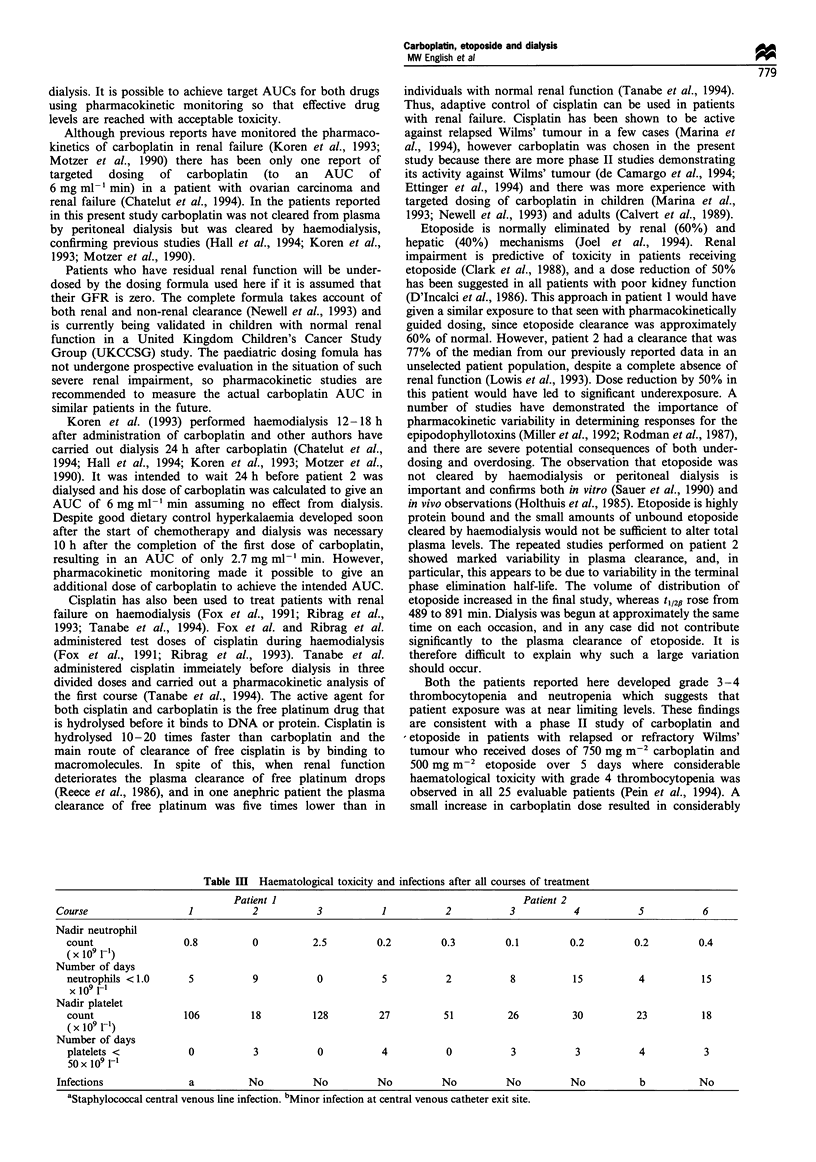
Two patients with relapsed Wilms' tumour and renal failure requiring dialysis were given carboplatin and etoposide by pharmacokinetically guided dosing. The target area under the drug plasma concentration vs time curve (AUC) was 6 mg ml-1 min for carboplatin and 18 and 21 mg ml-1 min for etoposide. On course 1 measured AUCs of carboplatin and etoposide were 6 and 20 mg ml-1 min for patient 1 and 6 and 21 mg ml-1 min for patient 2 respectively. Peritoneal dialysis did not remove carboplatin or etoposide from the plasma, however carboplatin but not etoposide was cleared by haemodialysis. Therapy with carboplatin and etoposide is possible in children and adults with renal failure who require dialysis, but in this situation pharmacokinetic monitoring is essential.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calvert A. H., Newell D. R., Gumbrell L. A., O'Reilly S., Burnell M., Boxall F. E., Siddik Z. H., Judson I. R., Gore M. E., Wiltshaw E. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989 Nov;7(11):1748–1756. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- Chatelut E., Rostaing L., Gualano V., Vissac T., De Forni M., Ton-That H., Suc J. M., Houin G., Canal P. Pharmacokinetics of carboplatin in a patient suffering from advanced ovarian carcinoma with hemodialysis-dependent renal insufficiency. Nephron. 1994;66(2):157–161. doi: 10.1159/000187794. [DOI] [PubMed] [Google Scholar]
- D'Argenio D. Z., Schumitzky A. A program package for simulation and parameter estimation in pharmacokinetic systems. Comput Programs Biomed. 1979 Mar;9(2):115–134. doi: 10.1016/0010-468x(79)90025-4. [DOI] [PubMed] [Google Scholar]
- D'Incalci M., Rossi C., Zucchetti M., Urso R., Cavalli F., Mangioni C., Willems Y., Sessa C. Pharmacokinetics of etoposide in patients with abnormal renal and hepatic function. Cancer Res. 1986 May;46(5):2566–2571. [PubMed] [Google Scholar]
- Ettinger L. J., Gaynon P. S., Krailo M. D., Ru N., Baum E. S., Siegel S. E., Hammond G. D. A phase II study of carboplatin in children with recurrent or progressive solid tumors. A report from the Childrens Cancer Group. Cancer. 1994 Feb 15;73(4):1297–1301. doi: 10.1002/1097-0142(19940215)73:4<1297::aid-cncr2820730427>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Fox J. G., Kerr D. J., Soukop M., Farmer J. G., Allison M. E. Successful use of cisplatin to treat metastatic seminoma during cisplatin-induced acute renal failure. Cancer. 1991 Oct 15;68(8):1720–1723. doi: 10.1002/1097-0142(19911015)68:8<1720::aid-cncr2820680812>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Harland S. J., Newell D. R., Siddik Z. H., Chadwick R., Calvert A. H., Harrap K. R. Pharmacokinetics of cis-diammine-1,1-cyclobutane dicarboxylate platinum(II) in patients with normal and impaired renal function. Cancer Res. 1984 Apr;44(4):1693–1697. [PubMed] [Google Scholar]
- Holthuis J. J., Van de Vyver F. L., van Oort W. J., Verleun H., Bakaert A. B., De Broe M. E. Pharmacokinetic evaluation of increasing dosages of etoposide in a chronic hemodialysis patient. Cancer Treat Rep. 1985 Nov;69(11):1279–1282. [PubMed] [Google Scholar]
- Koren G., Weitzman S., Klein J., Moselhy G. Comparison of carboplatin pharmacokinetics between an anephric child and two children with normal renal function. Med Pediatr Oncol. 1993;21(5):368–372. doi: 10.1002/mpo.2950210512. [DOI] [PubMed] [Google Scholar]
- Lowis S. P., Pearson A. D., Newell D. R., Cole M. Etoposide pharmacokinetics in children: the development and prospective validation of a dosing equation. Cancer Res. 1993 Oct 15;53(20):4881–4889. [PubMed] [Google Scholar]
- Marina N. M., Rodman J., Shema S. J., Bowman L. C., Douglass E., Furman W., Santana V. M., Hudson M., Wilimas J., Meyer W. Phase I study of escalating targeted doses of carboplatin combined with ifosfamide and etoposide in children with relapsed solid tumors. J Clin Oncol. 1993 Mar;11(3):554–560. doi: 10.1200/JCO.1993.11.3.554. [DOI] [PubMed] [Google Scholar]
- Marina N. M., Wilimas J. A., Meyer W. H., Jones D. P., Douglass E. C., Pratt C. B. Refining therapeutic strategies for patients with resistant Wilm's tumor. Am J Pediatr Hematol Oncol. 1994 Nov;16(4):296–300. [PubMed] [Google Scholar]
- Merimsky O., Reider-Groswasser I., Wigler N., Chaitchik S. Encephalopathy in ifosfamide-treated patients. Acta Neurol Scand. 1992 Nov;86(5):521–525. doi: 10.1111/j.1600-0404.1992.tb05136.x. [DOI] [PubMed] [Google Scholar]
- Miller A. A., Tolley E. A., Niell H. B., Stewart C. F., Griffin J. P. Pharmacodynamics of three daily infusions of etoposide in patients with extensive-stage small-cell lung cancer. Cancer Chemother Pharmacol. 1992;31(2):161–166. doi: 10.1007/BF00685105. [DOI] [PubMed] [Google Scholar]
- Motzer R. J., Niedzwiecki D., Isaacs M., Menendez-Botet C., Tong W. P., Flombaum C., Scher H. I., Bosl G. J. Carboplatin-based chemotherapy with pharmacokinetic analysis for patients with hemodialysis-dependent renal insufficiency. Cancer Chemother Pharmacol. 1990;27(3):234–238. doi: 10.1007/BF00685719. [DOI] [PubMed] [Google Scholar]
- Newell D. R., Eeles R. A., Gumbrell L. A., Boxall F. E., Horwich A., Calvert A. H. Carboplatin and etoposide pharmacokinetics in patients with testicular teratoma. Cancer Chemother Pharmacol. 1989;23(6):367–372. doi: 10.1007/BF00435838. [DOI] [PubMed] [Google Scholar]
- Newell D. R., Pearson A. D., Balmanno K., Price L., Wyllie R. A., Keir M., Calvert A. H., Lewis I. J., Pinkerton C. R., Stevens M. C. Carboplatin pharmacokinetics in children: the development of a pediatric dosing formula. The United Kingdom Children's Cancer Study Group. J Clin Oncol. 1993 Dec;11(12):2314–2323. doi: 10.1200/JCO.1993.11.12.2314. [DOI] [PubMed] [Google Scholar]
- Pein F., Tournade M. F., Zucker J. M., Brunat-Mentigny M., Deville A., Boutard P., Dusol F., Gentet J. C., Legall E., Mechinaud F. Etoposide and carboplatin: a highly effective combination in relapsed or refractory Wilms' tumor--a phase II study by the French Society of Pediatric Oncology. J Clin Oncol. 1994 May;12(5):931–936. doi: 10.1200/JCO.1994.12.5.931. [DOI] [PubMed] [Google Scholar]
- Pinkerton C. R., Rogers H., James C., Bowman A., Barbor P. R., Eden O. B., Pritchard J. A phase II study of ifosfamide in children with recurrent solid tumours. Cancer Chemother Pharmacol. 1985;15(3):258–262. doi: 10.1007/BF00263897. [DOI] [PubMed] [Google Scholar]
- Reece P. A., Stafford I., Russell J., Gill P. G. Reduced ability to clear ultrafilterable platinum with repeated courses of cisplatin. J Clin Oncol. 1986 Sep;4(9):1392–1398. doi: 10.1200/JCO.1986.4.9.1392. [DOI] [PubMed] [Google Scholar]
- Ribrag V., Droz J. P., Morizet J., Leclercq B., Gouyette A., Chabot G. G. Test dose-guided administration of cisplatin in an anephric patient: a case report. Ann Oncol. 1993 Sep;4(8):679–682. doi: 10.1093/oxfordjournals.annonc.a058624. [DOI] [PubMed] [Google Scholar]
- Rodman J. H., Abromowitch M., Sinkule J. A., Hayes F. A., Rivera G. K., Evans W. E. Clinical pharmacodynamics of continuous infusion teniposide: systemic exposure as a determinant of response in a phase I trial. J Clin Oncol. 1987 Jul;5(7):1007–1014. doi: 10.1200/JCO.1987.5.7.1007. [DOI] [PubMed] [Google Scholar]
- Sauer H., Füger K., Blumenstein M. Modulation of cytotoxicity of cytostatic drugs by hemodialysis in vitro and in vivo. Cancer Treat Rev. 1990 Sep;17(2-3):293–300. doi: 10.1016/0305-7372(90)90060-s. [DOI] [PubMed] [Google Scholar]
- Tanabe N., Goto M., Morita H., Gotu T., Inagaki J., Yamanaki N., Kimura K. Pharmacokinetics of cis-diammine-dichlor-platin in a hemodialysis patient. Cancer Invest. 1991;9(6):629–635. doi: 10.3109/07357909109039874. [DOI] [PubMed] [Google Scholar]
- de Camargo B., Melaragno R., Saba e Silva N., Mendonça N., Alvares M. N., Morinaka E., Marques A., Cusato M. P. Phase II study of carboplatin as a single drug for relapsed Wilms' tumor: experience of the Brazilian Wilms' Tumor Study Group. Med Pediatr Oncol. 1994;22(4):258–260. doi: 10.1002/mpo.2950220409. [DOI] [PubMed] [Google Scholar]


